Learning Objectives
This is an intermediate to advanced level course. After completing this course,
mental health professionals will be able to:
- Describe the core symptoms of ADHD.
- Explain how ADHD is a disorder of executive functioning and self-regulation.
- Discuss associated impairments and comorbid psychiatric disorders.
- Explain the typical developmental course and demographic distribution of ADHD.
- List the various etiologies that contribute to the development of ADHD.
- Describe cognitive disengagement syndrome (formerly sluggish cognitive tempo), a second attentional disorder.
The materials in this course are based on the most accurate information available to the author at the time of writing. The field of ADHD grows daily, and new information may emerge that supersedes these course materials. This course will equip clinicians to have a basic understanding of the nature of ADHD, the history of the disorder, its causes, and its associated disorders and impairments. This course is adapted from the relevant chapters contained in Barkley, R. A. (2015), Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment (4th ed.). New York: Guilford Press, from his rating scales of sluggish cognitive tempo (Barkley, 2016) and from his more recent book, Barkley, R. A. (2022). Treating Children and Adolescents with ADHD: What Every Clinician Needs to Know. New York: Guilford Press.
Outline
- Introduction
- A Brief History of ADHD
- Description and Diagnosis of ADHD
- Inattention
- Hyperactive-Impulsive Behavior (Disinhibition)
- Situational and Contextual Factors
- Associated Developmental Impairments
- Diagnostic Criteria and Related Issues
- Issues Pertaining to DSM-5-TR Criteria
- Is ADHD a “Real” Disorder?
- Epidemiology of ADHD
- Prevalence
- Sex Differences
- Socioeconomic Differences
- Ethnic/Cultural/National Issues
- Developmental Course and Adult Outcomes
- Comorbid Psychiatric Disorders
- Oppositional, Conduct, and Antisocial Disorders
- Anxiety and Mood Disorders
- Tic Disorders and Tourette’s Disorder
- Associated Develomental Problems
- Motor Incoordination
- Impaired Academic Functioning
- Reduced Intelligence
- Social Problems
- Health Outcomes
- ADHD and Early Mortality
- ADHD and Estimated Life Expectancy
- Etiologies
- Neurological Factors
- Neuropsychological Studies
- Neurological Studies
- Neurotransmitter Deficiencies
- Pregnancy and Birth Complications
- Genetic Factors
- Family Aggregation Studies
- Adoption Research
- Twin Studies
- Molecular Genetic Research
- Environmental Toxins
- Psychosocial Factors
- Summary
- Cognitive Disengagement Syndrome (Formerly Sulggish Cognitive Tempo) (CDS/SCT) – A Second Attention Disorder Often Comorbid with ADHD
- What Do We Know About the Nature of CDS Compared to ADHD?
- The Best Symptoms for Identifying CDS
- The Symptom Dimensions Are Distinct from ADHD
- CDS/SCT Symptoms Are Moderately Related to ADHD-Inattention, but Not Hyperactivity-Impulsivity
- CDS/SCT Symptoms Are Evident and Valid in Other Cultures/Countries
- Demographic Differences between CDS and ADHD
- Differences in Cognitive Functioning
- Overlap of ADHD and CDS
- Patterns of Comorbidity
- Domains of Impairment
- Etiology
- What is the Underlying Mental Dysfunction in CDS?
- Diagnosing CDS
- Treatment of CDS
- Conclusions
Introduction
This course provides an overview of the nature of Attention Deficit Hyperactivity Disorder, briefly considers its history, describes its primary symptoms, reinterprets these as evidence of deficits in executive functioning and self-regulation, discusses its developmental course and outcomes, and discusses its causes. It also contrasts a new, second attention disorder known as cognitive disengagement syndrome, or CDS (formerly sluggish cognitive tempo) that is often comorbid with ADHD, against what is known about ADHD to show its myriad differences from ADHD. Once considered a subtype of ADHD, CDS is increasingly being recognized as a second disorder of attention that requires distinct management from ADHD. Current critical issues related to these matters will be raised along the way. Given the thousands of scientific papers on these topics, this course must, of necessity, concentrate on the most important topics in this literature. The author’s theoretical model of executive functioning (Barkley, 2012) and its application to ADHD are presented in a separate course on executive functioning, but are also touched upon in this course and his other courses on child and adult ADHD and its management.
A Brief History of ADHD
Literary references to individuals having serious problems with inattention, hyperactivity, and poor impulse control date back to Shakespeare, who made reference to a malady of attention in King Henry VIII. A hyperactive child was the focus of a German poem, “Fidgety Phil,” by physician Heinrich Hoffman (see Stewart, 1970). William James (1890), in his Principles of Psychology, described a normal variant of character that he called the “explosive will” that resembles the difficulties experienced by those who today are called ADHD.
The history of ADHD in the medical literature is represented in the figure below. As shown in the lower right hand corner, by 2017 there were more than 300,000 scientific reports on ADHD and as of this writing (early 2023) that figure is now 1,050,000 citations in the medical literature. Clearly ADHD has become a well-studied condition.
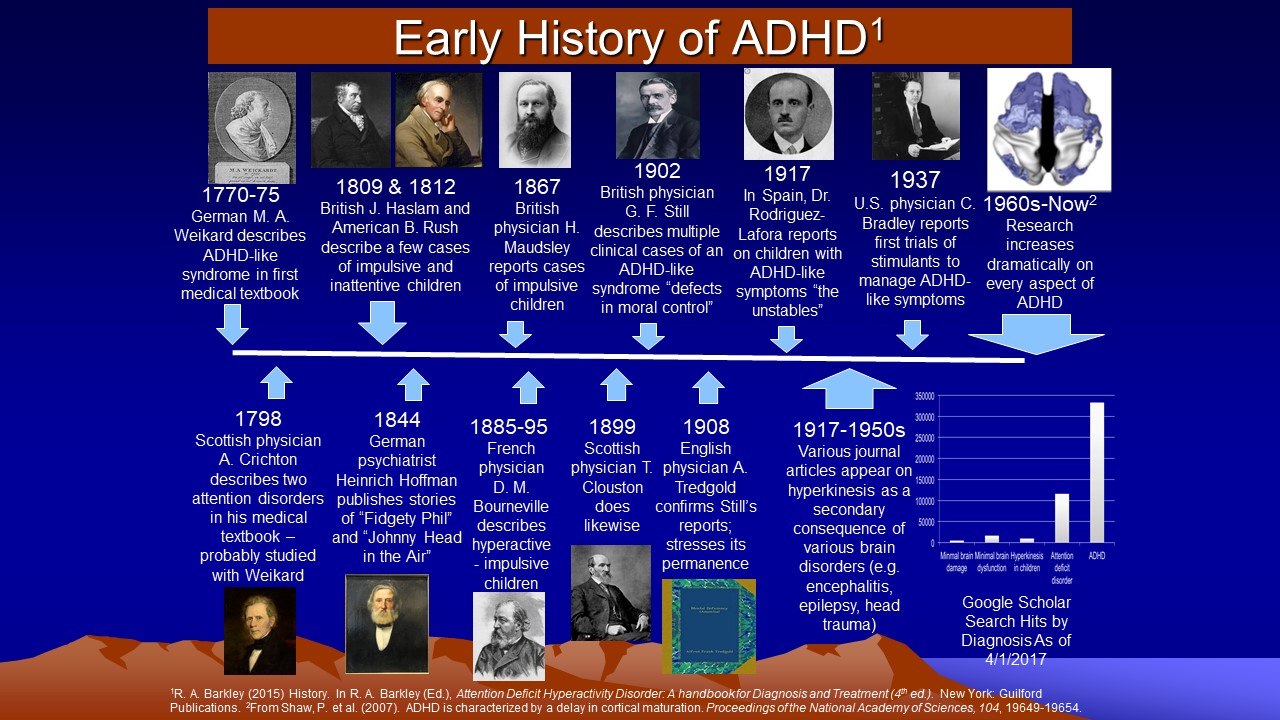
The first paper in the medical literature on disorders of attention such as ADHD is a short chapter on this topic in a medical textbook initially published anonymously by Melchior Adam Weikard in 1775 (Barkley & Peters, 2012). Weikard was a prominent German physician who described symptoms of distractibility, poor persistence, impulsive actions, and inattention more generally quite similar to the symptoms used today to describe the inattention associated with ADHD. This text was followed by that of the Scottish physician Alexander Crichton in 1798, who provided even more detailed descriptions of this sort of inattention as well as identifying a second disorder of attention thought to involve low power to focus attention (Palmer & Finger, 2001). Further serious clinical interest in ADHD did not occur again until the appearance of three lectures by the English physician George Still (1902) before the Royal Academy of Physicians.
Still reported on a group of 20 children in his clinical practice whom he defined
as having a deficit in “volitional inhibition” (p. 1,008) that led
to a “defect in moral control” (p. 1,009) over their own behavior.
Described as aggressive, passionate, lawless, inattentive, impulsive, and overactive,
many of these children today would be diagnosed not only as ADHD but also as
having oppositional defiant disorder (ODD). Still’s observations were
quite astute, describing many of the associated features of ADHD that would
come to be corroborated in research over the next century: (1) an overrepresentation
of male subjects (ratio of 3:1 in Still’s sample); (2) high comorbidity
with antisocial conduct and depression; (3) an aggregation of alcoholism, criminal
conduct, and depression among the biological relatives; (4) a familial predisposition
to the disorder, likely of hereditary origin; (5) yet with the possibility of
the disorder also arising from acquired injury to the nervous system.
Interest in these children arose in North America around the time of the great encephalitis epidemics of 1917-1918. Children surviving these brain infections had many behavioral problems similar to those comprising contemporary ADHD. These cases and others known to have arisen from birth trauma, head injury, toxin exposure, and infections gave rise to the concept of a brain-injured child syndrome, often associated with intellectual disability, that would eventually become applied to children manifesting these same behavior features but without evidence of brain damage or retardation. This concept evolved into that of minimal brain damage, and eventually minimal brain dysfunction (MBD), as challenges were raised to the original label in view of the dearth of evidence of obvious brain injury in most cases.
By the 1950s-1970s, focus shifted away from etiology and toward the more specific
behavior of hyperactivity and poor impulse control characterizing these children,
reflected in labels such as “hyperkinetic impulse disorder” or “hyperactive
child syndrome”. The disorder was thought
to arise from cortical overstimulation due to poor thalamic filtering of stimuli
entering the brain. Despite a continuing belief among clinicians and researchers
of this era that the condition had some sort of neurological origin, the larger
influence of psychoanalytic thought held sway. And so, when the second edition
of the Diagnostic and Statistical Manual of Mental Disorders (DSM-II) appeared,
all childhood disorders were described as “reactions,” and the hyperactive
child syndrome became “hyperkinetic reaction of childhood” (American
Psychiatric Association, 1968).
The recognition that the disorder was not caused by brain damage seemed to
follow a similar argument made somewhat earlier by the prominent child psychiatrist
Stella Chess (1960). It set off a major departure between professionals
in North America and those in Europe that continues, to a lessening extent,
to the present. Europe continued to view hyperkinesis for most of the latter
half of the 20th century as a relatively rare condition of extreme overactivity
often associated with intellectual disability or evidence of organic brain damage.
This discrepancy in perspectives has been converging over the last decades, as is
evident in the similarity of the DSM-IV criteria (see below) with those of ICD-10
(World Health Organization, 1994). Nevertheless, the manner in which clinicians
and educators view the disorder remains quite disparate; in North America, Canada,
and Australia, such children have ADHD, a developmental disorder, whereas in
Europe they are viewed as having conduct problem or disorder, a behavioral disturbance
believed to arise largely out of family dysfunction and social disadvantage.
By the 1970s, research emphasized the problems with sustained attention and impulse control in addition to hyperactivity. Douglas (1972, 1980, 1983) theorized that those with the disorder had three major deficits: (1) the investment, organization, and maintenance of attention and effort; (2) the ability to inhibit impulsive behavior; and (3) the ability to modulate arousal levels to meet situational demands; plus an unusually strong inclination to seek immediate reinforcement. Douglas’s emphasis on attention along with the numerous studies of attention, impulsiveness, and other cognitive sequelae that followed, eventually led to renaming the disorder as attention deficit disorder (ADD) in 1980 (DSM-III; American Psychiatric Association, 1980). Significant, historically, was the distinction in DSM-III between two types of ADD: those with hyperactivity and those without it. Little research existed at the time on the latter subtype that would have supported such a distinction being made in an official and increasingly prestigious diagnostic taxonomy. Yet, in hindsight, this bald assertion led to valuable research on the differences between these two supposed forms of ADD that otherwise would never have taken place. That research may have been fortuitous, as it may be leading to the conclusion that a subset of those having ADD without hyperactivity may actually be exhibiting a separate, distinct, and qualitatively unique disorder rather than a subtype of ADHD; one tentatively named sluggish cognitive tempo (SCT) which is now believed to be a qualitatively separate yet overlapping disorder of attention (Becker et al., 2022).
Even so, within a few years of the creation of the label ADD, concern arose
that the important features of hyperactivity and impulse control were being
de-emphasized, when in fact they were critically important to differentiating
the disorder from other conditions and to predicting later developmental risks. In 1987, the disorder was renamed
attention-deficit hyperactivity disorder in DSM-III-R (American Psychiatric
Association, 1987), and a single list of items incorporating all three symptoms
was specified. Also important here was the placement of the condition of ADD
without hyperactivity, renamed undifferentiated attention-deficit disorder,
in a separate section of the manual from ADHD with the specification that insufficient
research existed to guide in the construction of diagnostic criteria for it
at that time.
During the 1980s, reports focused instead on problems with motivation generally, and an insensitivity to response consequences specifically. Research was demonstrating that under conditions of continuous reward, the performances of ADHD children were often indistinguishable from normal children on various lab tasks, but when reinforcement patterns shifted to partial reward or to extinction (no reward) conditions, children with ADHD showed significant declines in their performance. It was also observed that deficits in the control of behavior by rules characterized these children.
Over the next decade, researchers employed information-processing paradigms to study ADHD and found that problems in perception and information-processing were not so evident as were problems with motivation and response inhibition. The problems with hyperactivity and impulsivity also were found to form a single dimension of behavior, which others described as “disinhibition.” All of this led to the creation of two separate but highly correlated lists of symptoms and thresholds for ADHD when the DSM-IV was published later in the decade (American Psychiatric Association, 1994; again in 2001); one for inattention and another for hyperactive-impulsive behavior. Unlike its predecessor, DSM-III-R, the establishment of the inattention list once again permitted the diagnosis of a subtype of ADHD that consisted principally of problems with attention (ADHD predominantly inattentive type). It also permitted, for the first time, the distinction of a subtype of ADHD that consisted chiefly of hyperactive-impulsive behavior without significant inattention (ADHD, predominantly hyperactive-impulsive type). Children having significant problems from both item lists, which constitute the majority of patients, were titled ADHD combined type.
All this has now been superseded by the development and publication of DSM-5-TR (American Psychiatric Association, 2022). The DSM-5-TR contains several changes discussed in more detail below (see “Diagnostic Criteria and Related Issues”). Suffice to say here that while the same symptoms remain as in DSM-IV, clarifications of them for older teens and adults have been added in parentheses next to each symptom; the age of onset has been increased to age 12; a lower symptom threshold of five will be required for adults (instead of the traditional six used with children); and the subtypes are eliminated, given that research over the prior 18 years did not find them reliable, stable over development nor valid, and hence not useful. ADHD is now recognized as a single disorder varying in severity across its two highly related symptom dimensions rather than comprised of three distinct types of a disorder. Clinicians will now simply specify which set of symptoms is more predominant by qualifying the diagnosis with a “presentation,” such as predominantly inattentive presentation.
Healthy debate continues to the present over the core deficit(s) in ADHD with increasing weight being given to problems with behavioral inhibition, self-regulation, and the related domain of executive functioning, as well as to delay aversion (difficulty waiting for events) and cognitive-energetic mechanisms (Faraone et al., 2024). The symptoms of inattention may actually be evidence of impaired working memory and not perceptual, filtering, or selection (input) problems. Likewise, interest continues into the other attention disorder identified in the last 30 years that is composed primarily of a distinct form of inattention, formerly called sluggish cognitive tempo (SCT), but now termed Cognitive Disengagement Syndrome (CDS; Becker et al, 2022). It is comprised of daydreaming, staring, slow processing, lethargy, and hypoactivity within the larger condition of ADHD. CDS (does not appear in DSM-5-TR given that far more research is needed on this condition in order to justify it being identified as a new and officially sanctioned psychiatric disorder. Yet research at the moment seems well on the way to eventually doing so (see second half of this course).
Description and Diagnosis of ADHD
The Core Symptoms
Research employing factor analysis has repeatedly identified two distinct behavioral dimensions underlying the various behavioral problems (symptoms) thought to characterize ADHD in children, while three factors appear to arise by adulthood. The two childhood dimensions have been identified across various ethnic and cultural groups, including Native American children, and represent inattention and hyperactive-impulsive behavior. In adults, a small verbal impulsivity dimension emerges that is somewhat separable from the other two. In contrast, symptoms of CDS form a separate dimension from those of ADHD, though partially correlated with them (especially the ADHD inattention dimension). This is some of the evidence used to argue that CDS is likely a distinct disorder from ADHD yet one that can be comorbid with it. Unlike ADHD, the symptoms of CDS do not appear to decline with age, have different family and demographic correlates, and manifest a different pattern of comorbidity with other psychiatric disorders in contrast to ADHD.
Inattention
Attention represents a multidimensional construct implying that several qualitatively distinct problems with attention eventually may be found. The dimension impaired in ADHD reflects an inability to sustain attention or persist at tasks or play activities, to remember and follow through on rules and instructions, and to resist distractions while doing so. I have elsewhere argued that this dimension more likely reflects problems with executive functioning, especially working memory than just poor attention, per se, and evidence is becoming available to support this contention. Parents and teachers frequently complain that these children do not seem to listen as well as they should for their age, cannot concentrate, are easily distracted, fail to finish assignments, are forgetful, and change activities more often than others. The same is true of adults with ADHD. Research employing objective measures corroborates these complaints about children through observations of more “off-task” behavior, less work productivity, greater looking away from assigned tasks (including television), less persistence at tedious tasks such as continuous performance tasks, being slower and less likely to return to an activity once interrupted, less attentiveness to changes in the rules governing a task, and being less capable of shifting attention across tasks flexibly. This inattentive behavior distinguishes these children from those with learning disabilities or other psychiatric disorders and does not appear to be a function of other disorders often comorbid with ADHD (anxiety, depression, or oppositional and conduct problems).
Hyperactive-Impulsive Behavior (Disinhibition)
As with attention, inhibition is a multidimensional construct, and thus various qualitatively distinct forms of inhibitory impairments may eventually be found in children. The problems with inhibition seen in ADHD are thought to involve voluntary or executive inhibition of prepotent or dominant responses rather than impulsiveness that may be more motivationally controlled, as in a heightened sensitivity to available reward (reward-seeking) or to excessive fear. Some evidence suggests that an excess sensitivity to reward or to sensation-seeking may be more associated with the severity of conduct disorder or psychopathy than with the severity of ADHD. Evidence is less clear about deficits in automatic or involuntary inhibition, as in eye-blinking or negative priming, being associated with ADHD.
More specifically, children with ADHD manifest difficulties with excessive activity level and fidgetiness, less ability to stay seated when required; greater touching of objects, moving about, running, and climbing than other children; playing noisily; talking excessively; acting impulsively; interrupting others’ activities; and being less able than others to wait in line or take turns in games. Parents and teachers describe them as acting as if driven by a motor, incessantly in motion, always on the go, and unable to wait for events to occur. Research objectively documents them to be more active than other children, to have considerable difficulties with stopping an ongoing behavior, to talk more than others, to interrupt others’ conversations, to be less able to resist immediate temptations and delay gratification, and to respond too quickly and too often when they are required to wait and watch for events to happen, as is often seen in impulsive errors on continuous performance tests. Although less frequently examined, these differences in activity and impulsiveness have been found between children with ADHD and those with learning disabilities. Mounting evidence further shows that these inhibitory deficits are not merely a function of other psychiatric disorders that may overlap with ADHD.
Interestingly, research shows that the problems with inhibition arise first (at age 3 to 4 years old) ahead of those related to inattention (at age 5 to 7 years old), and of those of SCT that may arise even later (ages 8 to 10 years old). Whereas the symptoms of disinhibition in the DSM item lists seem to decline with age until early adulthood (age 30), perhaps owing to their heavier weighting with hyperactive than impulsive behavior, those of inattention remain relatively more stable during the elementary grades. But even those symptoms eventually decline by adolescence and further until early adulthood, though not to normal levels in most cases. Why the inattention arises later than the disinhibitory symptoms and does not decline when the latter do over development remains an enigma. But it likely has to do with the fact that the inattention symptom list reflects the broader domain of executive deficits that may take more time to emerge in development yet prove highly persistent once apparent. As noted above, it could also simply reflect the different weightings of symptoms in the DSM. Those of hyperactivity may be more typical of preschool to early school-age children and are over-represented in the DSM list while those reflecting inattention may be more characteristic of school-age children and adults. Another explanation comes from the theoretical model described in my course on executive functioning in which inhibition and the two types of working memory (nonverbal and verbal) emerge at separate times in development.
Situational and Contextual Factors
The symptoms comprising ADHD are greatly affected in their level of severity by a variety of situational and task-related factors. Douglas (1972) commented on the greater variability of task performances made by ADHD children compared to the control group. Many others since then have found that excessive variability in behavior and task performance is commonplace in ADHD. The finding is especially common in measures of reaction time.
A number of other factors influence the ability of children with ADHD to sustain their attention to task performance, control their impulses to act, regulate their activity level, and/or to produce work consistently. Research dating back several decades shows that the performance of ADHD children is worse: (1) later in the day than earlier; (2) in greater task complexity such that organizational strategies are required (3) when restraint is demanded; (4) under low levels of stimulation; (5) under more variable schedules of immediate consequences in the task; (6) under longer delay periods prior to reinforcement availability; and (7) in the absence of adult supervision during task performance.
Besides the aforementioned factors, which chiefly apply to task performance, variability has also been documented across more macroscopic settings. For instance, children with ADHD are most problematic in their behavior when persistence in work-related tasks is required (i.e., chores, homework, etc.) or where behavioral restraint is necessary, especially in settings involving public scrutiny (i.e., in church, in restaurants, when a parent is on the phone, etc.) than in free play situations. Although they will be more disruptive when their fathers are at home than during free play, children with ADHD are still rated as much less problematic when the father is at home than in most other contexts. Fluctuations in the severity of ADHD symptoms have also been documented across a variety of school contexts. In this case, contexts involving task-directed persistence and behavioral restraint (classroom) are the most problematic, with significantly fewer problems posed by contexts involving less work and behavioral restraint (i.e., at lunch, in hallways, at recess, etc.), and even fewer problems being posed during special events (i.e., field trips, assemblies, etc.).
Associated Developmental Impairments
Children with ADHD often demonstrate deficiencies in many other cognitive abilities. Among these, are difficulties with: (1) physical fitness, gross and fine motor coordination, and motor sequencing (Meachon, Schaider, & Alpers, 2025); (2) speed of color naming; (3) verbal and nonverbal working memory and mental computation; (4) story recall; (5) planning and anticipation; (6) verbal fluency and confrontational communication; (5) effort allocation ; (6) developing, applying, and self-monitoring organizational strategies ; (7) the internalization of self-directed speech; (8) adhering to restrictive instructions; and (9) self-regulation of emotion. The latter difficulties with emotional control may be especially salient in children having ADHD with comorbid oppositional defiant disorder (ODD) and may well explain the high risk for developing ODD in most cases of ADHD. Several studies have also demonstrated that ADHD may be associated with less mature or diminished moral development. Many of these cognitive difficulties appear to be specific to ADHD and are not a function of its commonly comorbid disorders such as learning disabilities, depression, anxiety, or oppositional/conduct disorder.
The commonality among most or all of these seemingly disparate abilities is that all have been considered to fall within the domain of “executive functions” in the field of neuropsychology, or “metacognition” and self-regulation in developmental psychology, or to be affected by these functions. All seem to be mediated by the frontal cortex, and particularly the prefrontal lobes. Executive functions have been defined as those neuropsychological processes that permit or assist with human self-regulation, which itself has been defined as any behavior by a person that modifies the probability of a subsequent behavior by that person so as to alter the probability of a later consequence. By classifying cognitive actions or thinking as private behavior, one can understand how these private self-directed, cognitive (executive) actions fall within the definition of human self-regulation – they are private behaviors (cognitive acts) that modify other behaviors so as to alter the likelihood of later consequences for the individual. And, by appreciating the role of the frontal lobes generally, and the prefrontal cortex particularly, in these executive abilities, it is easy to see why researchers have repeatedly observed that ADHD arises out of some developmental disturbance or dysfunction of this brain region. (For more information on EF, see my course on Executive Functioning on this website.)
ADHD as a Disorder of Executive Functioning (EF) and Self-Regulation (SR)
The diagnostic criteria for ADHD are based on the disorder's most obvious behavioral symptoms. Viewed through that thin lens, as shown above, ADHD is surely a disorder comprised of inattention, impulsivity, and hyperactivity. But calling ADHD an attention disorder is like referring to autism spectrum disorder as "hand flapping, stereotyped movement, or odd behavior disorder." If we look at ADHD through a much thicker lens, we find it is far more than a set of obvious behaviors. Underneath those surface symptoms, ADHD is actually a disorder of self-regulation, making it more accurately self-regulation deficit disorder (SRDD). I am not the first to make this claim. The highly esteemed Canadian psychologist Virginia Douglas asserted 40 years ago (1980, 1988) that ADHD was a disorder of self-control, although she did not clearly define operationally what that term meant and what mental functions people employ for self-control that were deficient in people with ADHD. Now we recognize that self-regulation relies on executive function and its underlying brain networks. Therefore, ADHD could also be called EFDD. The reason I prefer the term SRDD is that it is the obvious and repeated failure to demonstrate self-regulation that is so apparent to those with ADHD, their families, and clinicians who are trying to evaluate and manage it. The deficits in executive function create that phenotype, but they are not so visible in the patient with ADHD, being largely private or mental activities, especially by adulthood. For instance, patients with ADHD may repeatedly forget to take their car keys when leaving and thus lock themselves out of their own home, may forget why they went into a room to get something, or forget that they agreed to meet someone for a meeting over lunch. These are obvious problems, yet the underlying deficit in verbal and nonverbal working memory and the governance of action plans by the sense of time giving rise to them remain unseen to others. The label SRDD is a useful reminder for clinicians that what you are seeing in those with ADHD is a heterogeneous set of wide-ranging, impairing problems with the executive functions and the self-regulation they provide.
Even though most or all investigators today recognize that executive functions involve mental abilities necessary for goal-directed action, there is still plenty of disagreement on the exact definition of executive function (20–30 definitions and counting), on what makes a mental function executive in nature, and on just how many functions fall under this umbrella (3–33 at last count!). The widespread idea that executive functions involve those cognitive abilities needed for goal-directed action, and thus enable an intentional stance toward the future, remains too vague for an operational definition. I address that critical problem shortly. What is important here to understand if you are to accurately diagnose and effectively treat those with ADHD is what abilities qualify as executive functions and how they operate in disrupting daily adaptive functioning.
Self-Regulation and the Development of Executive Functions
There's a missing link between the neuroanatomical malformations and the cognitive and behavioral symptoms associated with ADHD. That link is provided by viewing ADHD symptoms as executive function deficits. But to understand what executive function is, we first have to come up with an operational definition of just what constitutes an executive function. And then show how the seven major executive functions meet that definition. That solution comes from our understanding that executive function involves self-regulation.
B. F. Skinner and others have defined self-regulation as the self-direction of actions that are intended to modify subsequent behavior in order to alter the likelihood of a delayed (future) consequence. In my theory, an executive function is defined as a specific type or form of self-directed action. Here, then, is our operational definition. Cognitive or behavioral actions directed at oneself in order to change a subsequent behavior in an attempt to alter the future are, by definition, executive in nature. Cognitive and behavioral actions that are not self-directed for such purposes are not executive. We can usefully define the seven major executive functions as seven major types of actions-to-the-self that serve to modify subsequent behavior and thereby strive to change future events for that individual. I have further proposed that each EF is a human behavior or cognitive action initially directed toward the external world early in human development. It will eventually become self-directed and then progressively internalized (privatized) to form a largely mental self-directed activity – something done in the conscious mind. The specific case of private self-speech illustrates this more general process. In it, children start by directing speech out loud to the external environment generally and others specifically. They then enter a phase where they direct their speech at themselves, even when no one is in that context with them – yet it is still external speech; it is observable. Then gradually children internalize such self-directed speech. Privatize is more accurate, which is to say that this process involves the brain inhibiting peripheral neural activity and muscle movements while still activating the speech centers of the brain. Eventually, this gradual process of privatization reaches a point where the self-speech can't be observed publicly at all. Children now have a mind’s voice that only they can hear. I have argued that this illustrates the more general process by which all seven executive functions develop.
1. At first, children's actions are directed at the world around them. Infants have not yet developed EFs, so for instance when they learn to speak, they talk out loud to and about their surroundings, especially to other people in their environment.
2. Next, they direct their actions back at themselves, most of which may be observable. For instance, children talk to themselves even when no one else is present.
3. Subsequently, they internalize these self-directed actions through a process of inhibition of the associated peripheral movements while activating relevant brain regions and networks. For instance, self-speech gradually becomes quieter, involving barely audible whispers, then just facial movement, then subvocal actions, and finally suppression of the oral musculature. This progression is what we see in second- and third- graders who talk to themselves while doing math worksheets: Their mouths are moving, as if whispering to themselves, but they're making no audible sound, perhaps also while touching their fingers to aid their counting.
4. Finally, children can engage in these actions to themselves without visible peripheral motor and vocal movements. In the case of self-speech, the movements of the face, larynx, and diaphragm are being largely inhibited while the central speech centers of the brain are activated. Both speech and gesture in the example above of the second grader doing math will eventually be peripherally inhibited while remaining centrally activated in the brain and thus become a cognitive form of executive function – a mind tool for self-regulation. The self-directed actions are now occurring within the brain, but the associated nerve signals are not emitted into the spinal cord. Now these actions are internal and private. In our example, children can now talk to themselves in their mind without anyone seeing or hearing the speech. Children of this age often announce to their parents the discovery that there is a voice in their head. An entirely mental or cognitive form of behavior and self-regulation has now emerged. Thinking, in this case self-speech, then serves to govern motor actions, such as behavior toward goals.
5. Even later in development, people may create external cues to further assist the self-governing activities they are doing in their mind. For instance, as written language is acquired, people learn to write notes to themselves (“to do" lists) as yet another method of self-regulation using self-speech. Or they may place nonverbal cues, such as objects or pictures, in useful locations in their visual or sensory fields to further aid the stimulus control of their private forms of self-regulation.
6. This newly emerging private self can now mentally test out various ideas without engaging in their external or public performances and thereby avoid experiencing the real-world consequences that would have occurred with those publicly executed counterparts. Private or mental simulation of possible action plans is now possible, allowing for the natural selection of the most optimal among them while the mistaken ones die in our place, as Karl Popper once noted. In the case of self-speech, this means older children or teens can rehearse mentally what they want to say later publicly to improve their eventual public verbal performances. This can also be done for various motor activities using private visual-motor rehearsals.
A plurality of researchers identified at least seven executive functions. These are self-awareness, inhibition, nonverbal and verbal working memory, emotional self-regulation, self-motivation, and planning/problem solving (or manipulation of mental representations). My theory argues that all of these are forms of self-directed actions and all emerge via the same general developmental process noted earlier. Therefore, each executive function can be redefined by the action to the self that is involved in it:
- Self-directed attention (self-awareness)
- Self-restraint (volitional inhibition)
- Self-directed sensory-motor actions (visual imagery or nonverbal working memory)
- Self-directed speech (verbal working memory)
- The self-direction of emotions (emotional self-regulation), and thereby
- Self-motivation, and eventually
- Self-directed play (usually mental manipulations for planning and problem solving)
Over development, the maturation of these executive functions allows children greater degrees of freedom from being controlled purely by external events and others in the moment to becoming fully independent and self-controlling entities. That is because what is controlling their behavior is changing from early childhood to adulthood as represented in these four transitions from external to self (internal) control:
- From control by external events to self-control via mental representations (self-speech, visual imagery, etc.)
- From control by others to control by the self (using mental self-directed executive functions)
- From the present or now to the mentally conjectured future
- From small, immediate rewards (gratification) to delayed, larger rewards
In ADHD, the delayed and disrupted development of all seven executive functions greatly interferes with these extremely important transitions in what sources are regulating one’s behavior – the immediate and external world or the self and mental foresight.
Clinically, I've found it useful to explain the executive functions and their hierarchical development by referring to them—especially for children--simply as the mind's mirror, brakes, eye, voice, heart, fuel tank, and playground, respectively. All this is where the child or teen with ADHD is delayed in development. That leads to an equally useful principle to explain to parents and others. Children with ADHD have an executive age (EA) that is significantly below their chronological age (CA), and thus one cannot expect or demand them to self-regulate the way that typical peers are able to do. EFDD = CA – EA. The corollary of that idea is to reduce our expectations to match the child’s executive age and make necessary accommodations in the environment that support the child’s behavior and performance, thus making the child less impaired if not less ADHD. The extent of this lag in executive age will vary across children with ADHD, perhaps ranging from 20% to 45% below their chronological age. But that is not so important as realizing that the lag exists, is substantial, isn’t going away anytime soon, and requires accommodations. Of course, there are much greater clinical implications of the EF-SR theory to consider, which I do throughout this course. Yet even this one idea about delayed executive age is incredibly valuable to parents and teachers in understanding children and teens with ADHD and making accommodations for them.
The self-directed actions comprising the executive functions are essential for the contemplation of a hypothetical future -- essentially a goal. That hypothetical future is then juxtaposed against the present, which can lead to both the formation of an intention or goal and the plan to attain it. Thus, as many other experts have said, executive function is future directed. But lacking in such statements is the key point -- the executive functions are self-directed actions for behavioral self-modification so as to improve one’s future.
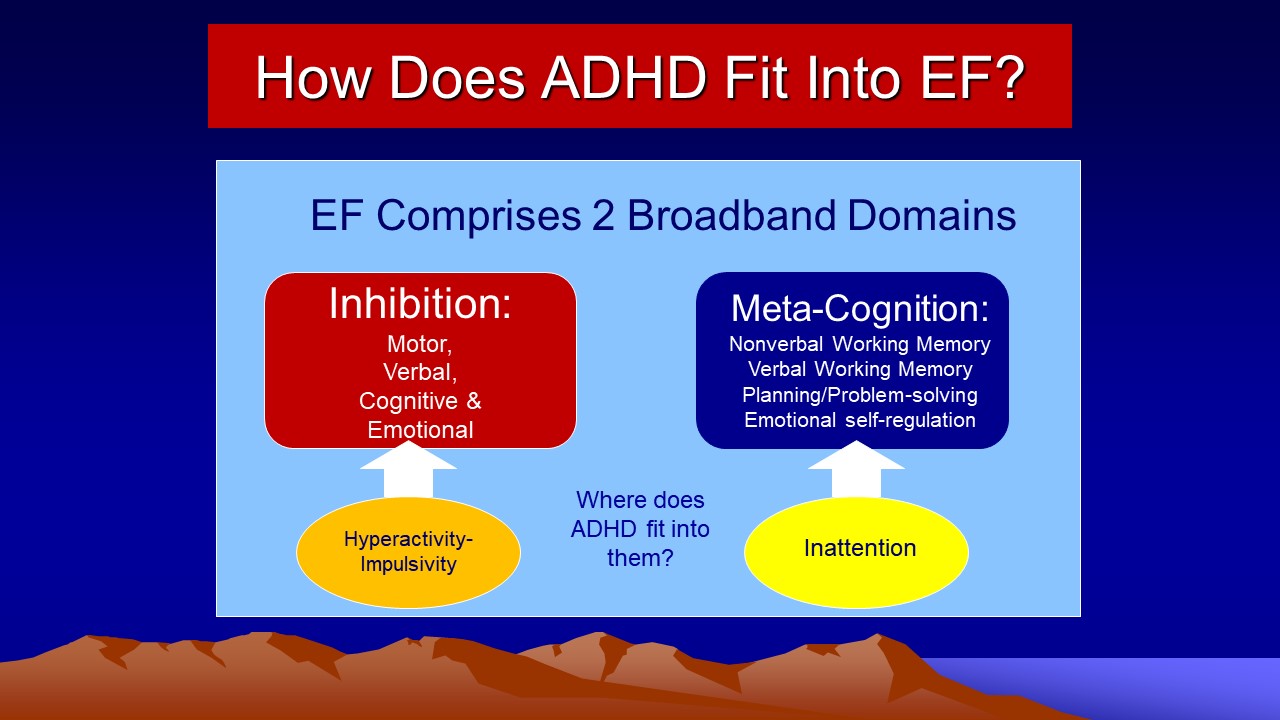
So just how do the classic DSM-5-TR symptoms of ADHD fit into this framework of ADHD as a disorder of EF? The Figure below illustrates the solution.
Diagnostic Criteria and Related Issues
Issues Pertaining to DSM-5-TR Criteria
The most recent diagnostic criteria for ADHD are defined in DSM-5-TR (American Psychiatric Association, 2022). These diagnostic criteria are some of the most rigorous and most empirically derived criteria ever available in the history of clinical diagnosis for this disorder. They were derived from various committees of some of the leading experts in the field who worked on this and on earlier DSMs, from literature reviews of ADHD, and from an informal survey of empirically derived rating scales assessing the behavioral dimensions related to ADHD by the committee. They were also derived from statistical analyses of the results of field trials of the items conducted for DSM III-R, DSM-IV, and most recently, field trials and analyses of large existing datasets conducted for DSM-5-TR.
Despite its empirical basis and recent revision, the DSM criteria continue to have some problems. As noted earlier, evidence is mounting that the predominantly inattentive type (DSM-IV), now Presentation (DSM-5-TR), of ADHD (ADHD-PI) may be comprised of a rather heterogeneous mix of children, many of whom are formerly Combined Presentations who outgrew enough hyperactive symptoms to no longer qualify for that Combined categorization. But it is becoming evident that a subset of PI cases have a qualitatively different disorder of attention and cognitive processing. Their symptoms generally consist of problems with daydreaming, staring, mental spaciness, lethargy, hypoactivity, and sluggishness. This subset is probably not a subtype of ADHD but may represent a separate disorder manifesting a sluggish cognitive style and selective attention deficit; having less comorbidity with oppositional and conduct disorder; having greater comorbidity with anxiety and depression; demonstrating a more passive or withdrawn pattern of social relationships; perhaps having memory retrieval problems; and, owing to their lower level of impulsiveness, probably having a different, more benign, developmental course. I believe the subset having hypoactivity, lethargy, and sluggish cognitive tempo should be set aside as a separate disorder from ADHD. (See the second half of this course on SCT.)
Are the two separate symptom lists important rather than the one combined list used in DSM-III-R? Perhaps, but this is unsure because these dimensions are highly correlated with each other and also have substantial shared genetic overlap. In the field trial for DSM-IV significant levels of inattention mainly predicted additional problems with completing homework that were not as well predicted by the hyperactive-impulsive behavior. Otherwise, the latter predicted most of the other areas of impairment studied in this field trial. Other studies find that childhood symptoms of hyperactivity are related to adverse adolescent outcomes, such as antisocial behavior, substance abuse, and school disciplinary actions such as suspensions/expulsions. Symptoms of inattention seem to be primarily predictive of impairment in academic achievement, particularly reading, and school performance. Severity of hyperactive-impulsive behavior is often found to be the dimension of ADHD that more strongly predicts later conduct disorder and so risk for various forms of substance use and abuse. A study suggests that adolescent inattention, however, may contribute further to the risk for tobacco use beyond that risk contributed by the severity of conduct disorder alone. For these and other reasons, DSM-5-TR now presents ADHD as a single disorder within the human population that can vary in severity along its two inter-related dimensions and thus accounts for some of the heterogeneity in clinical cases, with some having higher levels of one dimension than the other. These differing cases are now recognized as representing differences in “presentation” but not in typing or in the disorder itself.
Another critical issue only partially addressed in DSM-5-TR is how well the diagnostic thresholds set for the two symptom lists for children apply to age groups outside of those used in the earlier DSM-IV field trial (ages 4 to 16 years old, chiefly). This concern arises out of the well-known findings that the behavioral items comprising these lists, particularly those for hyperactivity, decline significantly with age. Applying the same threshold across such a declining developmental slope could produce a situation where a larger percentage of young preschool aged-children (ages 2 to 3 years old) would be inappropriately diagnosed as ADHD (false positives), whereas a smaller than expected percentage of adults would meet the criteria (false negatives). Support of just such a problem with using these criteria for adults was found in several studies collecting norms for DSM-IV item lists on large samples of adults, ages 17 to 84 years old. The threshold needed to place an individual at the 93rd percentile for that person’s age group declined to four of nine inattention items and five of nine hyperactive-impulsive items for ages 17 to 29 years old, which then fell to four of nine on each list for the 30- to 49-year-old age group, then to three of nine on each list for those 50 years old and older. Additional evidence also supports the use of a lower threshold for diagnosis with adults (typically four symptoms per list). For these reasons, the DSM-5-TR committee entertained dropping the symptom threshold to four of nine symptoms on each list, but eventually settled on using five of nine, likely so as not to increase the prevalence of adult ADHD too much through these adjustments to the diagnostic criteria.
This notion of changing symptom thresholds with age raises another critical
issue for developing diagnostic criteria for ADHD, and this is the appropriateness
of the content of the item set for different developmental periods. Inspection
of the item lists used in DSM-5-TR suggests that the items for inattention may have a wider developmental
applicability across school-age ranges of childhood and even into adolescence
and young adulthood. Those for hyperactive-impulsive behavior, in contrast,
seem much more applicable to young children and less appropriate or not at all
to older teens and adults. Recall from above that the symptoms
of inattention remain more stable across middle childhood into early adolescence,
whereas those for hyperactive-impulsive behavior decline more steeply
over this same course. Although this may represent a true developmental decline
in the severity of the latter symptoms, and possibly in the severity and prevalence
of ADHD itself, it could also represent an illusory developmental trend. That
is, it might be an artifact of using more preschool-focused items for hyperactivity
and more school-age-focused items for inattention.
Barkley, et al. (2008) have identified seven symptoms that are not part of DSM-5-TR yet are better at discriminating adults with ADHD from those with other disorders or a general population sample. Hyperactive symptoms were not helpful in such discrimination but symptoms of poor executive functioning were so. These symptoms were:
- Often easily distracted by extraneous stimuli;
- Often makes decisions impulsively;
- Often has difficulty stopping activities or behavior when they should do so;
- Often starts a project or task without reading or listening to directions carefully;
- Often shows poor follow-through on promises or commitments made to others;
- Often has trouble doing things in their proper order or sequence; and
- Often more likely to drive a motor vehicle much faster than others (Excessive speeding). (If person has no driving history, substitute: “Often has difficulty engaging in leisure activities or doing fun things quietly.”)
Having four of these seven symptoms was sufficient to accurately distinguish adults with ADHD from adults with other disorders and from a general community sample.
Unfortunately, the DSM-5-TR committee working on ADHD elected not to incorporate any new items such as those above into the ADHD symptom list, either to capture the problems with inhibition better or to provide better items for identifying adults with ADHD. Instead, the DSM-5-TR now has parenthetical clarifications inserted next to each of the previous 18 DSM-IV items to make them more applicable to adolescents and adults with the disorder. Time will tell if those clarifications achieve that purpose.
Also of concern was the absence of any requirement in the DSM-IV for the symptoms to be corroborated by someone that has known the patient well, such as a parent, sibling, long-time friend, or partner. In the case of adults who are self-referred to professionals, this oversight proved problematic. For instance, available evidence suggests that ADHD children, teens and young adults significantly under-report the severity of their symptoms relative to the reports of parents. This pattern continues until about age 27 to 30 when the reverse begins to become apparent. There is good reason that self-awareness might be limited by this disorder, at least until early adulthood. For these reasons, the DSM-5-TR now recommends that patient self-reports be corroborated by someone who knows the patient well in making a diagnosis of ADHD at any age.
These issues are not merely academic. My colleagues and I have been involved in follow-up research with ADHD children into their adulthood and have been impressed at the chronicity of impairments created by the disorder despite an apparent decline in the percentage of cases continuing to meet diagnostic criteria and an apparent decline in the severity of the symptoms used in these criteria. Recently, we found that if the formerly ADHD children, who are now adults, are interviewed using the DSM criteria, just 5% of them report sufficient symptoms to receive the diagnosis, a figure nearly identical to that for the New York longitudinal studies. If instead, the parents are interviewed, this figure rises to 46% – a nine-fold difference in persistence of disorder as a function of reporting source. If instead of the recommended DSM symptom threshold, one substitutes a developmentally referenced criterion (the 98th percentile) based on same-age control adults, then 12% of the probands now have the disorder as adults based on self-reports, while the figure climbs to 66% based on parental reports. Whose reports of current functioning are more valid? We addressed this by examining the relationship of self-reports and parent-reports to various domains of major life activities and outcomes (education, occupational functioning, friendships, crime, etc.). Parent reports made a substantially larger contribution to nearly all outcome domains, and did so for more such domains than did self-reports, suggesting that parental reports probably have greater validity. The higher rates of disorder they reported at outcome are probably the more accurate ones. Such adjustments for age and source of reporting, however, do not correct for the potentially increasing inappropriateness of the item sets for this aging sample, and so it is difficult to say how many of those not meeting these adjusted criteria may still have had the disorder.
A different issue pertains to whether or not the criteria should be adjusted for the gender of the children being diagnosed. Research evaluating these and similar item sets demonstrates that male youngsters display more of these items and to a more severe degree than do female youngsters in the general population. Given that the majority of children in the DSM field trial were boys, the symptom threshold chosen in the DSM-IV is most appropriate to male children. This results in girls having to meet a higher threshold relative to other girls to be diagnosed as ADHD than do boys relative to other boys. Gender-adjusted thresholds for children would seem to be in order to address this problem, yet this would evaporate the currently disproportionate male-to-female ratio of 3:1 found across studies (see below). However, by adulthood, there appear to be no significant gender differences in ADHD symptoms (see Barkley, 2011a).
The requirement of an age of onset for ADHD symptoms (7 years old) in the DSM-IV diagnostic criteria came under early attack from its own field trial (a longitudinal study), and a review of this criterion from historical, empirical, and pragmatic perspectives. Although I have suggested that age 18 might serve as a better age of onset criterion for diagnosis because it captures more than 98% of all cases, the DSM-5-TR has decided to raise the threshold to age 12. This serves to partially correct the problem in DSM-IV, but may still leave 7%-10% of cases of ADHD ineligible for the diagnosis due to an age of onset later than this one. Because research shows that the age of onset has little relevance to diagnosing otherwise legitimate case of ADHD (Vater et al., 2024), I recommend that it be ignored in diagnosis.
A related potential problem for these criteria occurs in their failure to stipulate
a lower bound age group for giving the diagnosis below which no diagnosis should
be made. This is important because research on preschool children has shown
that a dimension of hyperactive-impulsive behavior separate from aggression
or defiant behavior does not seem to emerge until about the age of 3. Below this age, these behaviors cluster
together to form what has been called behavioral immaturity, externalizing problems,
or an under-controlled pattern of conduct. This implies that the symptoms of
ADHD may be difficult to distinguish from other early behavioral disorders until
at least 3 years old, and so this age might serve as a lower bound for diagnostic
applications.
Similarly, research implies that a lower bound of IQ (>50) might also be important, below which the nature of ADHD may be quite different. Minimal
research seems to exist that speaks to the issue of a discontinuity or qualitative
shift in the nature of ADHD in individuals below IQs of 50. Some indirect evidence
implies that this may occur, however. Children
who fall below this level of IQ may have a qualitatively different form of mental
retardation. This is inferred from findings that this group is over represented
for its position along a normal distribution and from findings that genetic
defects contribute more heavily to this subgroup. Given this shift in the prevalence
and causes of intellectual disability below this level of IQ, a similar state of
affairs might exist for the form of ADHD associated with it necessitating its
distinction from the type of ADHD that occurs in individuals above this IQ level.
Consistent with such a view have been findings that the percentage of positive
responders to stimulant medication falls off sharply below this threshold of
IQ.
Another issue pertinent to the above is the problem of the duration requirement
being set at six months. This has been chosen mainly out of tradition (earlier
DSMs) with no research support for selecting this particular length of time
for symptom presence. It is undoubtedly important that the symptoms be relatively
persistent if we are to view this disorder as a developmental disability rather
than arising purely from context or out of a transient, normal developmental
stage. Yet specifying a precise duration is difficult in the absence of much
research to guide the issue. Research on preschool-aged children might prove
helpful here, however. Such research has shown that many children aged 3 years old
or younger may have parents or preschool teachers who report concerns about
the activity level or attention of the children, yet these concerns have a high
likelihood of remission within 12 months. It would seem for preschoolers that the six-month
duration specified in the DSM-IV may be too brief, resulting in over-identification
of ADHD children at this age (false positives). However, this same body of research
found that for those children whose problems lasted at least 12 months or beyond
age 4, the behavior problems were highly persistent and predictive of
continuance into the school-age range. Such research suggests that the duration
of symptoms be set at 12 months or more.
The DSM requirement that the symptoms be demonstrated in at least two of three environments, so as to establish pervasiveness of symptoms, is new to this edition and is problematic. The DSM implies that two of three sources of information (parent, teacher, employer) must agree on the presence of the symptoms. This confounds settings with sources of information. The degree of agreement between parents and teacher for any dimension of child behavior is modest, often ranging between .30 and .50. This sets an upper limit on the extent to which parents and teachers are going to agree on the severity of ADHD symptoms and, thus, on whether or not the child has the disorder in that setting. Such disagreements among sources certainly reflect differences in the child’s behavior as a function of true differential demands of these settings. But they also reflect differences in the attitudes and judgments between different people. Insisting on such agreement may reduce the application of the diagnosis to some children unfairly as a result of such well-established differences between parent and teacher opinions. It may also create a confounding of the disorder with issues of comorbidity with oppositional defiant disorder (ODD). Parent-only-identified ADHD children may have predominantly ODD with relatively milder ADHD, whereas teacher-only-identified ADHD children may have chiefly ADHD and minimal or no ODD symptoms. Children identified by both parents and teachers as ADHD may, therefore, carry a higher likelihood of ODD. They may also simply reflect a more severe condition of ADHD than do the home- or school-only cases, being different in degree rather than in kind. Research is clearly conflicted on the matter. Considering that teacher information on children is not always obtainable or convenient, that parents can convey the essence of that information to clinicians, and that diagnosis based on parents’ reports will lead to a diagnosis based on teacher reports 90% of the time, one may infer that parent reports could suffice for diagnostic purposes for now. However, more recent evidence suggests that the best discrimination of ADHD children from other groups might be achieved by blending the reports of parents and teachers such that one counts the number of different symptoms endorsed across both sources of information.
Is ADHD a “Real” Disorder?
Social critics in prior decades and the Church of Scientology via its Citizens Commission on Human Rights routinely have charged that professionals have been too quick to label energetic and exuberant children as having a mental disorder. They also assert that educators may be using these labels as an excuse for simply poor educational environments. In other words, children who are hyperactive or ADHD are actually normal but are being labeled as mentally disordered because of parent and teacher intolerance or lack of love at home. If this were actually true, then we should find no differences of any cognitive, neurological, genetic, behavioral, or social significance between children so labeled and normal children. We should also find that the diagnosis of ADHD is not associated with any significant risks later in development for maladjustment within any domains of adaptive functioning, or social, occupational, or school performance. Furthermore, research on potential etiologies for the disorder should, likewise, come up empty-handed.
This is hardly the case, as evidence reviewed in this course attests. Differences between ADHD and normal children are too numerous to take these assertions of normality seriously. As will be shown later, substantial developmental risks await the child meeting clinical diagnostic criteria for the disorder, and certain potential etiological factors are becoming consistently noted in the research literature.
Conceding all of this, however, does not automatically entitle ADHD to be placed
within the realm of valid (“real”) disorders. Wakefield (1999) has
argued that disorders must meet two criteria to be viewed as valid: They must (1) engender
substantial harm to the individual or those around him or her, and (2) incur
dysfunction of natural and universal mechanisms that have been selected in an
evolutionary sense (have survival value). The latter criterion is simply the
definition of an adaptation as used in evolutionary biology. Disorders
are failures in adaptation that produce harm. In the case of psychology,
these universal mechanisms are psychological ones possessed by all normally
developing humans, regardless of culture. ADHD handily meets both criteria.
Those with ADHD, as described in the theory presented below, have significant
deficits in behavioral inhibition and inattention (the executive functions)
that are critical for effective self-regulation. And, those with ADHD experience
numerous domains of impairment (risks of harm) over the course of development, as will become
evident below.
Epidemiology of ADHD
Prevalence
The prevalence of ADHD varies across studies, at least in part due to different methods of selecting samples, the nature of the populations from which they are drawn (nationality or ethnicity, urban vs. rural, community vs. primary care settings, etc.), the criteria used to define ADHD (DSM criteria vs. rating scale cutoff), and certainly to the age range and sex composition of the samples. When only the endorsement of the presence of the behavior of hyperactivity (not the clinical disorder) is required from either parent or teacher rating scales, prevalence rates can run as high as 22% to 57%. This underscores the point made earlier that being described as inattentive or overactive by a parent or teacher does not in and of itself constitute a disorder in a child. When diagnostic criteria such as those in the DSM are used, prevalence rates are between 7% and 10% of children and 2.5% to 5.5% of adults.
The most accurate assessments of prevalence come from two large-scale studies. One was a meta-analysis of worldwide prevalence studies that reported an average of 5.5% of children, while an epidemiological study of U.S. adults as well as a worldwide prevalence study placed the prevalence at 3.4% to 4.4%.
Prevalence varies as a function of young age, male
gender, chronic health problems, family dysfunction, low socioeconomic status,
presence of a developmental impairment, and urban living.
As noted above in discussing DSM criteria, it may be that the declining prevalence of ADHD with age is partly due to the types of items contained in the DSM symptom list that are chiefly applicable to young children. This could create a situation where individuals remain impaired in the construct(s) comprising ADHD as they mature, such as inhibition and executive functioning, while outgrowing the symptom list for the disorder, resulting in an illusory decline in prevalence. This was noted in my Milwaukee follow-up study discussed above. Until more age-appropriate symptoms are studied for adolescent and adult populations, this issue remains unresolved.
Sex Differences
As noted above, sex appears to play a significant role in determining prevalence of ADHD within the childhood population, at least in children. On average, male children are between 2.5 and 5.6 times more likely than female children to be diagnosed as ADHD within epidemiological samples, with the average being roughly 3:1. This is not the case by adulthood where the ratio is closer to 1.5:1 or less. Within clinic-referred samples, the gender ratio can be considerably higher, suggesting that boys with ADHD are far more likely to be referred to clinics than girls. This is probably because boys are more likely to have a comorbid oppositional or conduct disorder.
Studies of clinic-referred girls often find that they are as impaired as clinic-referred boys with ADHD, have as much comorbidity, and may even have greater deficits in intelligence, according to reviews of sex differences in ADHD (Babinski, 2024). Some studies suggest these clinic-referred girls, at least as adolescents, may have more internalizing symptoms such as depression, anxiety, and stress; greater problems with teacher relationships; and poorer verbal abilities (vocabulary) in comparison to ADHD boys. Like the boys, girls with ADHD also manifest more conduct, mood, and anxiety disorders, have lower intelligence, and have greater academic achievement deficits than do control samples. Males with ADHD may have greater problems with cognitive processing speed than females, but these differences were no longer significant after controlling for severity of ADHD. No gender differences have been identified in executive functioning as measured by cognitive tests, with both genders being more impaired than control samples on such measures. In contrast, studies drawing their ADHD samples from the community find that girls are significantly less likely to have comorbid ODD and CD than boys with ADHD, do not have greater intellectual deficits than ADHD boys, yet may be as socially and academically impaired as boys with the disorder.
Socioeconomic Differences
Few studies have examined the relationship of ADHD to social class, and those
that have are not especially consistent. Trites
(1979), and later Szatmari (1992), both found that rates of ADHD tended to increase
with lower socioeconomic class. However, in his own study, Szatmari (Szatmari, et al., 1989) found that low socioeconomic status was no longer associated with
rates of ADHD when other comorbid conditions such as conduct disorder were
controlled. For now, it is clear that ADHD occurs across all socioeconomic levels.
Variations across social classes may be artifacts of the source used to define
the disorder or of the comorbidity of ADHD with other disorders related to social
class, such as oppositional defiant disorder and conduct disorder.
Ethnic/Cultural/National Issues
Early studies of the prevalence of hyperactivity, relying principally on teacher
ratings, found significant disparities across four countries (United States,
Germany, Canada, and New Zealand), ranging from 2% in girls and 9% in boys in
the United States to 9% in girls and 22% in boys in New Zealand. Similarly, O’Leary, Vivian, and Nisi (1985) found rates of hyperactivity
to be 3% in girls and 20% in boys in Italy using this same teacher rating scale
and cutoff score. However, this may have resulted from the use of a threshold
established on norms collected in the United States across these other countries,
where the distributions were quite different from those found in the United
States. Later studies, especially those using DSM criteria, have found the disorder
to exist across all countries studied to date.
Early studies found differences among ethnic groups in rates of hyperactivity within the United
States. Langsdorf, Anderson, Walchter, Madrigal, and Juarez
(1979) reported that almost 25% of African-American children and 8% of Latino-Americans
met a cutoff score on a teacher rating scale commonly used to define hyperactivity,
whereas Ullmann (cited in O’Leary, et al., 1985) reported rates of 24%
for African-American children and 16% for white Americans on a teacher rating
scale. Such differences, however, may arise in part
because of socioeconomic factors that are differentially associated with these
ethnic groups in the United States. Such psychosocial factors are strongly correlated
with aggression and conduct problems. As noted above, those factors no longer
make a significant contribution to the prevalence of ADHD when comorbidity for
other disorders is controlled. Doing the same within studies
of ethnic differences might well reduce or eliminate these differences in prevalence. In a recent survey of U.S. adults, Barkley (2011a) found no significant differences among ethnic groups except a slightly higher level of hyperactivity reported in Hispanic-Latino males. Thus, it would seem that ADHD exists in all ethnic groups studied
so far. Whether the differences in prevalence across these ethnic groups are
real or are a function of the source of information about the symptoms of ADHD
and, possibly, socioeconomic factors remains to be determined.
Developmental Course of ADHD and Adult Outcomes
Major follow-up studies of clinically referred hyperactive children have been ongoing during the last 25 years at five sites: Montreal, New York City, Iowa City, Los Angeles, and Milwaukee. Follow-up studies of children identified as hyperactive from a general population have also been conducted in the United States, New Zealand, and England, among other locales.
But before embarking on a summary of their results, some cautionary notes are
in order. The
differing entry/diagnostic criteria across follow-up studies must be kept in
mind in interpreting and cross-referencing their outcomes. Most studies selected
for children known at the time as “hyperactive.” Such children are
most likely representative of the course of the ADHD Combined Type from the
current DSM taxonomy. Even then, the degree of deviance of the samples on parent
and teacher ratings of these symptoms was not established at the entry point
in most of these studies. These studies also cannot be viewed as representing
the Inattentive subtype from DSM-IV or now the new CDS group of children and adults, for which no follow-up information is currently available.
The descriptions of clinic-referred ADHD children who are of similar age groups
to those in the follow-up studies but who are not followed over time may help
understand the risks associated with different points in development. However,
these may also be contaminated by cohort effects at the time of referral and
so can only be viewed as suggestive. Such cohort effects may be minor; that
is, adolescents with ADHD referred to clinics seem to have similar types and
degrees of impairment as ADHD children followed up to adolescence.
The average onset of ADHD symptoms, as noted earlier, is often in the preschool years, typically at ages 3 to 4 years old and, more generally, by entry into formal schooling. Yet, as noted earlier, only half of all ADHD cases have developed by age 7, while 93% have developed by age 12 and >98% by age 16.
Preschool-aged children who are perceived as difficult and resistant to control or who have inattentive and hyperactive behavior that persists for at least a year or more are highly likely to have ADHD and to remain so into elementary school years and even adolescence. Persistent cases seem especially likely to occur where parent-child conflict, greater maternal directiveness and negativity, and greater child defiant behavior exist. More negative temperament and greater emotional reactivity to events are also more common in preschool ADHD children. It is little wonder that greater parenting stress is associated with preschool ADHD children and seems to be at its highest relative to later age groups. Within the preschool setting, ADHD children will be found to be more often out of their seats, wandering the classroom, being excessively talkative and vocally noisy, and disruptive of other children’s activities.
By the time ADHD children move into the elementary-age range of 6 to 12 years old,
the problems with hyperactive-impulsive behavior are likely to continue
and to be joined now by difficulties with attention (executive functioning and
goal-directed persistence) and even greater impairment (Overgaard, et al., 2024). Difficulties with work completion and productivity,
distraction, forgetfulness related to what needs doing, lack of planning, poor
organization of work activities, trouble meeting time deadlines associated with
home chores, school assignments, and social promises or commitments to peers
are now combined with the impulsive, heedless, and disinhibited behavior typifying
these children since preschool age. Problems with oppositional and socially
aggressive behavior may emerge at this age in at least 40% to 70% of ADHD children.
By ages 8 to 12 years old, these early forms of defiant and hostile behavior may evolve further into symptoms of conduct disorder in 25% to 45% or more of all children with ADHD. Certainly by late childhood, most or all of the deficits in the executive functions are likely to be arising and interfering with adequate self-regulation. Not surprisingly, the overall adaptive functioning (self-sufficiency) of many ADHD children is significantly below their intellectual ability. This is also true of preschoolers with high levels of these externalizing symptoms. The disparity between adaptive functioning and age appropriate expectations (or IQ) may itself be a predictor of greater severity of ADHD as well as of greater risk for oppositional and conduct problems in later childhood. The disorder takes its toll specifically on self-care, personal responsibility, chore performance, trustworthiness, independence, appropriate social skills, and timeliness; and on moral conduct generally.
If ADHD is present in clinic-referred children, the likelihood is that 50%
to 80% will continue to have their disorder into adolescence, with most studies
supporting the higher figure. Using the same parent rating scales at both the childhood and
adolescent evaluation points, Fischer, et al. (1993a) were able to show that
inattention, hyperactive-impulsive behavior, and home conflicts declined by
adolescence. The hyperactive group showed far more marked declines than the
control group, mainly because the former were so far from the mean of the normative
group to begin with in childhood. Nevertheless, even at adolescence, the groups
remained significantly different in each domain, with the mean for the hyperactives
remaining two standard deviations or more above the mean for the controls. This
emphasizes the point made earlier that simply because severity levels of symptoms
are declining over development does not mean hyperactive children are necessarily
outgrowing their disorder relative to normal children. Like intellectual disability,
ADHD may need to be defined by a developmentally relative deficiency, rather
than an absolute one, that persists in most children over time.
The persistence of ADHD symptoms across childhood as well as into early adolescence appears, again, to be associated with the initial degree of hyperactive-impulsive behavior in childhood; the coexistence of conduct problems or oppositional hostile behavior; poor family relations and specifically, conflict in parent-child interactions; as well as with maternal depression, and duration of mental health interventions. These predictors have also been associated with the development and persistence of oppositional and conduct disorder into this age range.
Studies following large samples of clinic-referred children with hyperactivity (or ADHD) into adulthood are few in number. Only four follow-up studies have retained at least 50% or more of their original sample into adulthood and reported on the persistence of symptoms to that age. These are the Montreal study by Weiss, Hechtman, and their colleagues (see Weiss & Hechtman, 1993); the New York City study by Mannuzza, Klein, and colleagues (see Klein, et al., 2012; Mannuzza, Klein, Bessler, Malloy, & LaPadula, 1993, 1998; the Swedish study by Rasmussen and Gillberg (2001); and my research with Mariellen Fischer in Milwaukee (Barkley, et al., 2008; Barkley, Fischer, et al., 2002; Fischer, et al., 2002). The results regarding the persistence of disorder into young adulthood (middle 20s) are mixed but can be better understood as being a function of the reporting source and the diagnostic criteria used.
The Montreal study (N=103) found that two-thirds of their original sample (N=64;
mean age of 25 years) claimed to be troubled as adults by at least one or more
disabling core symptoms of their original disorder (restlessness, impulsivity,
or inattention) and that 34% had at least moderate to severe levels of hyperactive,
impulsive, and inattentive symptoms.
In Sweden (N=50), Rasmussen and Gillberg (2001) obtained similar results, with
49% of probands reporting marked symptoms of ADHD at age 22 compared to
9% of controls. Formal diagnostic criteria for ADHD, as in DSM-III or
later editions, were not employed at any of the outcome points in either study,
however. In contrast, the New York study has followed two separate cohorts
of hyperactive children using DSM criteria to assess persistence of disorder.
That study found that 31% of their initial cohort (N=101) and 43% of their second
cohort (N=94) met DSM-III criteria for ADHD by ages 16-23 (mean age=18.5 years)
(Gittelman, et al., 1985; Mannuzza, et al., 1991). Eight years later (mean
age=26 years) however, these figures fell to 8% and 4%, respectively (now using
DSM-III-R criteria) (Mannuzza, et al., 1993, 1998). At 30-year follow-up, the rate of ADHD diagnosed using DSM-IV criteria and by self-report was 22%. Those results might
imply that the vast majority of hyperactive children no longer qualify for the
diagnosis of ADHD by adulthood.
The interpretation of the relatively low rate of persistence of ADHD into adulthood,
particularly for the New York study, is clouded by at least two issues apart
from differences in selection criteria. One is that the source of information
about the disorder changed in all of these studies from that used at the childhood
and adolescent evaluations to that used at the adult outcome. At study
entry and at adolescence, all studies used the reports of others (parents and,
typically, teachers). By mid-adolescence, all found that the majority of
hyperactive participants (50% to 80%) continued to manifest significant levels of
the disorder (see above). In young adulthood (approximately age 26),
both the New York and Montreal studies switched to self-reports of disorder.
The rather marked decline in persistence of ADHD from adolescence to adulthood
could stem from this change in source of information. Indeed, the New
York study found this to be likely when, at late adolescence (mean age of 18 to 19 years old), they interviewed both the teenagers and their parents about psychiatric
status of the teens. There was a marked
disparity between the reports of parents and teens concerning the presence of
ADHD (11% vs. 27%; agreement 74%, Kappa=.19). Other research also suggests that
the relationship between older children’s (age 11) self-reports of externalizing
symptoms, such as those involved in ADHD, and those of parents and teachers
is quite low (r=.16-.32).
Thus, changing sources of reporting in longitudinal studies on behavioral disorders
could be expected to lead to marked differences in estimates of persistence
of those disorders.
The question obviously arises as to whose assessment of the proband is more
accurate. This would depend on the purpose of the assessment, but the
prediction of impairment in major life activities would seem to be an important
one in research on psychiatric disorders. Our Milwaukee study examined
these issues by interviewing both the participants and their parents about ADHD
symptoms at the young adult follow-up (age 21 years old). It then examined
the relationship of each source’s reports to significant outcomes in major
life activities (education, occupation, social, etc.) after controlling for
the contribution made by the other source. As noted earlier, another limitation
in the earlier studies may reside in the DSM criteria in that they grow less
sensitive to the disorder with age. Using a developmentally referenced
criterion (age comparison) to determine diagnosis may identify more cases than
would the DSM approach. As discussed earlier, the Milwaukee study found
that the persistence of ADHD into adulthood was heavily dependent on the source
of the information (self or parent) and the diagnostic criteria (DSM or developmentally
referenced). Self-report identified just 5% to 12% of probands as currently
ADHD (DSM-III-R) while parent reports placed this figure at 46% to 66%.
Using the DSM resulted in lower rates of persistence (5% for proband
reports and 46% for parents) while using a developmentally referenced
cutoff (98th percentile) yielded higher rates of persistence (12% by self-report
and 66% by parent reports). Parental reports appeared to have greater
validity in view of their greater contribution to impairment and to more domains
of current impairment than did self-reported information.
We concluded that past follow-up studies grossly underestimated the persistence
of ADHD into adulthood by relying solely on the self-reports of the probands and using DSM criteria at adulthood, criteria developed only for children. As discussed above, using a childhood threshold for symptoms – such as six – with adults may be inappropriate whereas a lower threshold of four symptoms may be more accurate in detecting the disorder in older age groups. For instance, at an age 27 follow-up, persistence of disorder was estimated to be 65% to 86% depending on the rigor of the definition of recovery.
Comorbid Psychiatric Disorders
Individuals diagnosed with ADHD are often found to have a number of other disorders besides their ADHD. What is known about comorbidity is largely confined to the Combined Type of ADHD. In community derived samples, up to 44% of ADHD children have at least one other disorder and 43% have at least two or more additional disorders. The figure is higher, of course, for children drawn from clinics. As many as 87% of clinically diagnosed ADHD children may have at least one other disorder and 67% have at least two other disorders. Results for adults with ADHD are nearly as high, with more than 80% having at least one disorder and more than 50% having two or more. The disorders likely to co-occur with ADHD are briefly described below.
Results for adults with ADHD are nearly as high, with more than 80% having at least one disorder and more than 50% having two or more. The disorders likely to co-occur with ADHD are shown in the next figure (from Barkley, 2022) and are briefly described below.
Oppositional, Conduct, and Antisocial Disorders
The most common comorbid disorders with ADHD are oppositional defiant disorder (ODD) and, to a lesser extent, conduct disorder (CD). Indeed, the presence of ADHD increases the odds of ODD/CD by 10.7-fold (95% Confidence Interval [CI] = 7.7-14.8) in general population studies (See my other course on this website on Oppositional Defiant Disorder). Studies of clinic-referred ADHD children find that between 54% and 67% will meet criteria for a diagnosis of ODD by age 7 or later. ODD is a frequent precursor to CD, a more severe and often (though not always) later occurring stage of ODD. The co-occurrence of CD with ADHD may be 20% to 50% in children and 44% to 50% in adolescents with ADHD. By adulthood, up to 26% may continue to have CD while 12% to 21% will qualify for antisocial personality disorder (ASPD). Similar or only slightly lower degrees of overlap are noted in studies using epidemiologically identified samples rather than those referred to clinics. ADHD, therefore, has a strong association with conduct problems and antisocial disorders, such as ODD, CD, and ASPD, and has been found to be one of the most reliable early predictors of these disorders. Recent longitudinal research suggests that severity of early ADHD is actually a contributing factor to risk for later ODD regardless of severity of early ODD, perhaps due to the problems with poor emotion (anger) regulation in ADHD noted above.
Familial associations among the disorders have also been consistently found, whether across boys and girls with ADHD or across Caucasian and African-American samples. This suggests some underlying causal connection among these disorders. Evidence from twin studies indicates a shared or common genetic contribution to the three disorders, particularly between ADHD and ODD. When CD occurs in conjunction with ADHD, it may represent simply a more severe form of ADHD having a greater family genetic loading for ADHD. Other research, however, also suggests a shared environmental risk factor may also account for the overlap of ODD and CD with ADHD beyond their shared genetics, that risk factor likely being family adversity generally and impaired parenting specifically. To summarize, ODD and CD have a substantial likelihood of co-occurring with ADHD with the risk for ODD/CD being mediated in large part by severity of ADHD and its family genetic loading and in part by adversity in the familial environment.
One of the strongest predictors of risk for substance use and abuse disorders (SUDs) among ADHD children upon reaching adolescence and adulthood is prior or co-existing CD or ASPD. Given the heightened risk for ODD/CD/ASPD in ADHD children as they mature, one would naturally expect a greater risk for SUDs as well. Yet, ADHD alone is a predictor of adolescent and adult alcohol and nicotine use and probably some illicit drug use as well.
Anxiety and Mood Disorders
The overlap of anxiety disorders with ADHD has been found to be 10% to 40% in clinic-referred children, averaging to about 25%. In longitudinal studies of ADHD children, however, the risk of anxiety disorders is no greater than in control groups at either adolescence or young adulthood but does rise to 33% of those whose ADHD persists to age 27. The disparity in findings for children is puzzling. Perhaps some of the overlap of ADHD with anxiety disorders in children is due to referral bias. General population studies of children, however, do suggest an elevated odds ratio of having an anxiety disorder in the presence of ADHD of 3.0 (95%CI = 2.1-4.3), with this relationship being significant even after controlling for comorbid ODD/CD. This implies that the two disorders may have some association apart from referral bias, at least in childhood. The co-occurrence of anxiety disorders with ADHD has been shown to reduce the degree of impulsiveness relative to those ADHD children without anxiety disorders. Some research suggests that the disorders are transmitted independently in families and so are not linked to each other in any genetic way. This may not be the case for the DSM-IV inattentive type of ADHD or what is now called SCT, where higher rates of anxiety disorders have been noted in some studies of these children, though not always, and in their first- and second-degree relatives again though not always. Regrettably, research on the overlap of anxiety disorders with ADHD has generally chosen to collapse across the types of anxiety disorders in evaluating this issue. Greater clarity and clinical utility from these findings might occur if the types of anxiety disorders present were to be examined separately.
The evidence for the co-occurrence of mood disorders, such as major depression or dysthymia (a milder form of depression), with ADHD is now fairly substantial. Between 15% and 75% of those with ADHD may have a mood disorder, though most studies place the association between 20% and 30%. The odds of having depression, given the presence of ADHD in general population samples, is 5.5 (95% CI 3.5-8.4). Some evidence also suggests that these disorders may be related to each other in that familial risk for one disorder substantially increases the risk for the other, particularly where ADHD is comorbid with CD. Similarly, a follow-up study of mine found a 26% risk of major depression among ADHD children by young adulthood, but this risk was largely mediated by the co-occurrence of CD. Current depression fell to 14% of the ADHD cases by adulthood. Likewise, a meta-analysis of general population studies indicated that the link between ADHD and depression was entirely mediated by the linkage of both disorders to CD. In the absence of CD, ADHD was not more likely to be associated with depression.
The comorbidity of ADHD with bipolar (manic-depressive) disorder is controversial. Some studies of ADHD children indicate that 10% to 20% may have bipolar disorder – a figure substantially higher than the 2% risk for the general population. Follow-up studies of hyperactive children, however, have not documented any significant increase in risk of bipolar disorder in children with ADHD followed into adulthood with rates typically close to those for the general population. However, that risk would have to exceed 7% or more for these studies to have sufficient power to detect any comorbidity. A four-year follow-up of ADHD children reported that 12% met criteria for bipolar disorder in adolescence. ADHD children without bipolar disorder do not have an increased prevalence of bipolar disorder among their biological relatives, while ADHD children with bipolar disorder do, suggesting that where the overlap occurs it may represent a familially distinct subset of ADHD.
Children and adolescents diagnosed with childhood bipolar disorder often have a significantly higher lifetime prevalence of ADHD, particularly in their earlier childhood years. Where the two disorders co-exist, the onset of bipolar disorder may be earlier than in bipolar disorder alone. In bipolar cases that start in adulthood, comorbidity with ADHD may be only 20% to 25% while it may average 62% and in some studies be as high as 80% to 97% in cases where bipolar disorder begins in childhood. Some of this overlap with ADHD may be partly an artifact of similar symptoms comprising the symptom lists used for both diagnoses (i.e., hyperactivity, distractibility, poor judgment, etc.). In any case, the overlap of ADHD with bipolar disorder appears to be unidirectional – a diagnosis of ADHD seems not to increase the risk for bipolar disorder, whereas a diagnosis of childhood bipolar disorder seems to dramatically elevate the risk of a prior or concurrent diagnosis of ADHD.
Tic Disorders and Tourette’s Disorder
Up to 18% of children may develop a motor tic in childhood that declines to a base rate of about 2% by mid-adolescence and less than 1% by adulthood. The risk for tics among children with ADHD is only slightly higher than this; about 20%. Tourette’s Disorder (TD), a more severe disorder involving multiple motor and vocal tics, occurs in less than 0.4% of the population. A diagnosis of ADHD does not appear to necessarily elevate these risks for a diagnosis of tics or Tourette’s Disorder, at least not in childhood or adolescence. Among clinic-referred adults diagnosed with ADHD, there may be a slightly greater occurrence of tic disorders (12%). In contrast, individuals with obsessive-compulsive disorder (OCD) or TD have a marked elevation in risk for ADHD, averaging 48% or more (range of 35% to 71%). Complicating matters is the fact that the onset of ADHD often seems to precede that of TD in cases of comorbidity.
Associative Developmental Problems
Apart from an increased risk for various psychiatric disorders, children and
teens with ADHD are also more likely to experience a substantial
array of developmental and health risks, discussed below. Far less is
known about the extent to which these correlated problems are evident in the
Inattentive Type, particularly the subgroup having problems with sluggish cognitive
tempo described above. The developmental and social problems most likely
to occur with ADHD are briefly listed in Table 1. (From Barkley, 2015).
Table 1: Summary of Impairments Likely to
be Associated with ADHD |
Cognitive
- Mild deficits in intelligence (approximately 7-10 points)
- Deficient academic achievement skills (range of 10-30 standard
score points)
- Learning disabilities: Reading (8%-39%), Spelling (12%-26%), Math
(12%-33%), and Handwriting (common but unstudied)
- Poor sense of time, inaccurate time estimation and reproduction
- Decreased nonverbal and verbal working memory
- Impaired planning ability
- Reduced sensitivity to errors
- Possible impairment in goal-directed behavioral creativity
Language
- Delayed onset of language (up to 35%, but not consistent)
- Speech impairments (10% to 54%)
- Excessive conversational speech (commonplace), reduced speech to
confrontation
- Poor organization and inefficient expression of ideas
- Impaired verbal problem-solving
- Co-existence of central auditory processing disorder (minority
but still uncertain)
- Poor rule-governed behavior
- Delayed internalization of speech (>30% delay)
- Diminished development of moral reasoning
Adaptive Functioning
- 10-30 standard score points behind normal
Motor Development
- Delayed motor coordination (up to 52%)
- More neurological "soft" signs related to motor coordination
and overflow movements
- Sluggish gross motor movements
Emotion
- Poor self-regulation of emotion
- Greater problems with frustration tolerance
- Under-reactive arousal system
School Performance
- Disruptive classroom behavior (commonplace)
- Underperforming in school relative to ability (commonplace)
- Academic tutoring (up to 56%)
- Repeat a grade (30% or more)
- Placed in one or more special education programs (30%-40%)
- School suspensions (up to 46%)
- School expulsions (10%-20%)
- Failure to graduate high school (10%-35%)
Task Performance
- Poor persistence of effort/motivation
- Greater variability in responding
- Decreased performance/productivity under delayed rewards
- Greater problems when delays are imposed within the task and as
they increase in duration
- Decline in performance as reinforcement changes from being continuous
to intermittent
- Greater disruption when non-contingent consequences occur during
the task
Medical/Health Risks
- Greater proneness to accidental injuries (up to 57%)
- Possible delay in growth during childhood
- Difficulties getting ready for bed and sleeping (up to 30%-60%)
- Greater driving risks: vehicular crashes and speeding tickets
- Greater medical expenses for family to bear
- Start sexual intercourse earlier as teens
- Greater risk of teen pregnancy (38%)
- Greater risk of sexually transmitted disease (16%)
|
Motor Incoordination
As a group, as many as 60% of ADHD, compared to up to 35% of normal children, may have poor motor coordination or developmental coordination disorder Meachon et al., 2025). Neurological examinations for "soft" signs related to motor coordination and motor overflow movements find ADHD children to demonstrate more such signs as well as generally sluggish gross motor movements than control children, including those with purely learning disabilities. These overflow movements have been interpreted as indicators of delayed development of motor inhibition.
Studies using tests of fine motor coordination such as balance, fine motor gestures, electronic or paper-and-pencil mazes, and pursuit tracking often find children with ADHD to be less coordinated in these actions. Simple motor speed, as measured by finger-tapping rate or grooved pegboard tests, does not seem to be as affected in ADHD as is the execution of complex, coordinated sequences of motor movements. The vast majority of the available research, therefore, supports the existence of deficits in motor control in those with ADHD, particularly when motor sequences must be performed.
Impaired Academic Functioning
The vast majority of clinic-referred children with ADHD have difficulties with school performance, most often under-productivity of their work. ADHD children frequently fall below normal or control groups of children on standardized achievement tests. Difficulties with academic performance are predicted more by inattention than hyperactive-impulsive symptoms, while disruptive behavior is related more to the latter than the former. These differences from typically developing children are likely to be found even in preschool-age children with ADHD, suggesting that the disorder may take a toll on the acquisition of academic skills and knowledge even before entry into first grade. This makes sense given that inattention symptoms are highly correlated with executive functions believed to be disrupted by ADHD and that such executive functions are also likely to be involved in some forms of academic achievement (i.e., working memory in mental arithmetic or spelling; internalized speech in reading comprehension; verbal fluency in oral narratives and written reports, etc.).
Between 19% and 26% of children with ADHD are likely to have any single type of learning disability, conservatively defined as a significant delay in reading, arithmetic, or spelling relative to intelligence and achievement in one of these three areas at or below the 7th percentile with over 45% having at least one or more LDs. If the criterion of simply two grades below grade level is used, then as many as 80% of ADHD children in late childhood (age 11) may have learning disorders. Studies suggest that the risk for reading disorders among ADHD children is 16% to 39%, while that for spelling disorders is 24% to 27%, and for math disorders, the risk is 13% to 33%.
While the finding that children with ADHD are more likely to have learning disabilities might imply a possible genetic link between the two disorders, more recent research shows that the two sets of disorders are transmitted independently in families. Some subtypes of reading disorders associated with ADHD may share a common genetic etiology. This may arise from the finding that early ADHD may predispose children toward certain types of reading problems, whereas early reading problems are not as likely to give rise to later symptoms of ADHD, though the two disorders may interact to reciprocally increase risk for the other over time. The picture is less clear for spelling disorders, where a common or shared genetic etiology to both ADHD and spelling disorder has been shown in a joint analysis of twin samples from London and Colorado. This may result from the fact that early spelling ability seems to be linked to the integrity of working memory that may be impaired in those with ADHD. Writing disorders have not received as much attention in research on ADHD, though handwriting deficits are often found among children with ADHD, particularly those having the Combined type of the disorder.
Rapport, Scanlan, and Denney (1999) provide some evidence for a dual pathway
model of the link between ADHD and academic underachievement. Briefly,
ADHD may predispose to academic underachievement through its contribution to
a greater risk for ODD/CD and conduct problems in the classroom more generally,
the net effect of which is to adversely impact productivity and general school
performance. But ADHD is also associated with cognitive deficits not only
in attention, but in general intelligence (see below) and working memory (see above),
all of which may have a direct and adverse impact on academic achievement.
Also supportive of this view are findings that it is more the inattention
dimension of ADHD that is most closely associated with academic achievement problems
than the hyperactive-impulsive dimension. According to this
dual pathway model, both pathways will require interventions if the marked association
of ADHD with school under-achievement is to be addressed.
A higher prevalence of speech and language disorders has also been documented in many studies of ADHD children, typically ranging from 30% to 64% of the samples. The converse is also true; children with speech and language disorders have a higher than expected prevalence of ADHD (approximately 30% to 58%), among other psychiatric disorders.
Reduced Intelligence
Clinic-referred ADHD children often have lower intelligence than control groups used in these same studies, particularly in verbal intelligence. Differences in IQ have also been found between hyperactive boys and their normal siblings. The differences found in these studies often range from 7 to 10 standard score points. Studies using both community samples and behavior-problem samples also have found significant negative associations between degree of ADHD and intelligence (rs = -.25-.35). In contrast, associations between ratings of conduct problems and intelligence in children are often much smaller or even non-significant, particularly when hyperactive-impulsive behavior is partialed out of the relationship. This implies that the relationship between IQ and ADHD is not likely to be a function of comorbid conduct problems. Much of the difference between ADHD and typically developing groups appears to be the result of EF deficits associated with ADHD.
Social Problems
The interpersonal behaviors of those with ADHD, as noted earlier, are often
characterized as more impulsive, intrusive, excessive, disorganized, engaging,
aggressive, intense, and emotional. And so they are “disruptive”
of the smoothness of the ongoing stream of social interactions, reciprocity,
and cooperation that is an increasingly important part the children’s
daily life with others.
Research finds that ADHD affects the interactions of children with their parents and, hence, the manner in which parents may respond to these children. Those with ADHD are more talkative, negative and defiant, less compliant and cooperative, more demanding of assistance from others, and less able to play and work independently of their mothers. Their mothers are less responsive to the questions of their children, more negative and directive, and less rewarding of their children’s behavior. Mothers of ADHD children have been shown to give both more commands as well as more rewards to their ADHD sons than daughters but also to be more emotional and acrimonious in their interactions with their sons. ADHD children and teens seem to be nearly as problematic for their fathers as their mothers. Contrary to what may be seen in normal mother-child interactions, the mother-child conflicts in ADHD children and teens may actually increase when fathers join the interaction, especially in hyperactive boys. Such increased maternal negativity and acrimony toward sons in these interactions has been shown to predict greater noncompliance in classroom and play settings and greater covert stealing away from home, even when the level of the child’s own negativity and parental psychopathology are statistically controlled in the analyses.
The negative parent-child interaction patterns also occur in the preschool age group and may be even more negative and stressful (to the parent) in this age range than in later age groups. With increasing age, the degree of conflict in these interactions lessens but remains deviant from normal into later childhood and adolescence. Negative parent-ADHD child interactions in childhood have been observed to be significantly predictive of continuing parent-teen conflicts eight to 10 years later in adolescence in families with ADHD children. Few differences are noted between the interactions of mothers of ADHD children with those children as compared to their interactions with the siblings of the ADHD children.
The presence of comorbid ODD is associated with the highest levels of interaction conflicts between parents and their ADHD children and adolescents. In a sequential analysis of these parent-teen interaction sequences, investigators have noted that it is the immediate or first lag in the sequence that is most important in determining the behavior of the other member of the dyad. That is, the behavior of each member is determined mainly by the immediately preceding behavior of the other member and not by earlier behaviors of either member in the chain of interactions. The interactions of the comorbid ADHD/ODD group reflected a strategy best characterized as “tit for tat” in that the type of behavior (positive, neutral, or negative) of each member was most influenced by the same type of behavior emitted immediately preceding it. Mothers of ADHD only and normal teens were more likely to utilize positive and neutral behaviors regardless of the immediately preceding behavior of their teens, characterized as a “be nice and forgive” strategy that is thought to be more mature and more socially successful for both parties in the long run. Even so, those with ADHD alone are still found to be deviant from normal in these interaction patterns even though less so than the comorbid ADHD/ODD group. The presence of comorbid ODD has also been shown to be associated with greater maternal stress and psychopathology as well as with marital difficulties.
These interaction conflicts in families with ADHD children are not limited to just parent-child interactions. Increased conflicts have been observed between ADHD children and their siblings relative to normal child-sibling dyads. Research on the larger domain of family functioning has shown that parents of ADHD children experience more parenting stress and decreased sense of parenting competence, increased alcohol consumption in parents, decreased extended family contacts, and increased marital conflict, separations, and divorce as well as maternal depression. Again, it is the comorbid association of ADHD with ODD or CD that is linked to even greater degrees of parental psychopathology, marital discord, and divorce than in ADHD-only children. Interestingly, Pelham and Lang (1993) have shown that the increased alcohol consumption in these parents is, in part, directly a function of the stressful interactions they have with their ADHD children.
Research has demonstrated that the primary direction of effects within these interactions is from child to parent rather than the reverse. That is, much of the disturbance in the interaction seems to stem from the effects of the child’s excessive, impulsive, unruly, noncompliant, and emotional behavior on the parent rather than from the effects of the parent’s behavior on the child. This was documented primarily through studies that evaluated the effects of stimulant medication on the behavior of the children and their interaction patterns with their mothers. Such research found that medication improves the compliance of those with ADHD and reduces their negative, talkative, and generally excessive behavior such that their parents reduce their levels of directive and negative behavior as well. These effects of medication are noted even in the preschool-aged group of children with ADHD as well as in those in late childhood, and in both genders of ADHD children. Besides a general reduction in the negative, disruptive, and conflictual interaction patterns of these children with parents resulting from stimulant medication, general family functioning also seems to improve when ADHD children are treated with stimulant medication.
None of this is to say that parental reactions to disruptive child behavior, parental skill and competence in child management and daily rearing, and parental psychological impairment are unimportant influences on children with ADHD. Evidence certainly shows that parental management, child monitoring, parental antisocial activity, maternal depression, father absence, and other parent and family factors are exceptionally important in the development of ODD, CD, major depression, and other disorders likely to be comorbid with ADHD. But it is to say, as the behavioral genetic studies below will strongly attest, that these are not the origins of the child’s impulsive, hyperactive, and inattentive behavior and the related deficits in executive functioning and self-regulation.
More recent research shows that some degree of the family conflicts in children with ADHD also results from the fact that their parents are more likely to have ADHD and that parental ADHD can interfere with typical parenting behavior, whether or not the child has ADHD. Parents with ADHD are less attentive and rewarding, more negative, and monitor their children less than do typical parents.
The patterns of disruptive, intrusive, excessive, negative, and emotional social interactions of ADHD children noted above in parent-child relations have been found to also occur in their interactions with teachers. It should come as no surprise, then, that those with ADHD receive more correction, punishment, censure, and criticism from their teachers than do other children, as well as more school suspensions and expulsions, particularly if they have ODD/CD.
In their social relationships, ADHD children experience substantial difficulties. They are less liked by other children, have fewer friends, and are overwhelmingly rejected as a consequence, particularly if they have comorbid conduct problems. Indeed, among such comorbid cases, up to 70% may be rejected by peers and have no reciprocated friendships by 4th grade. These peer relationship problems are not only the result of the more active, talkative, and impulsive actions of ADHD children, but are also a consequence of their greater emotional, facial, tonal, and bodily expressiveness, particularly anger, and more limited reciprocity in interactions, use of fewer positive social statements, more limited knowledge of social skills, and more negative physical behavior. Those with ODD/CD also prefer more sensation-seeking, fun-seeking, and trouble-seeking activities that further serve to alienate their normal peers. ADHD children seem to process social and emotional cues from others in a more limited and error-prone fashion, as if they were not paying as much attention to emotional information provided by others. Yet they do not differ in their capacity to understand the emotional expressions of other children. However, in those with comorbid ODD/CD, there may be a greater misperception of anger and a greater likelihood of responding with anger and aggression to peers than in normal children. Little wonder then that ADHD children perceive themselves as receiving less social support from peers (and teachers) than do normal children. The problems with aggression and poor emotion regulation are also evident in the sports behavior of ADHD children with their peers. Once more, stimulant medication has been observed to decrease these negative and disruptive behaviors toward teachers and peers, but may not result in any increase in more prosocial or positive initiatives toward peers.
Health Outcomes
The following is adapted from my article on the adverse health outcomes of ADHD (Barkley, 2020, The ADHD Report). Nearly 20 years ago, suggestive evidence began to arise that ADHD may have a detrimental impact on life expectancy, as I discussed in an editorial in this newsletter in the late 1990s. My concerns about ADHD adversely impacting life expectancy were based on what were then new findings from a longitudinal study at Stanford university initiated by Terman of gifted children followed their entire lifespan. The findings by Friedman and colleagues indicated that even among this gifted sample, children who placed below the bottom 25th percentile of the population in the personality trait known as Conscientiousness had a 7-8 year reduction in their lifespan compared to the remainder of their sample. Conscientiousness refers to the use of one’s conscience in making decisions about one’s actions and their consequences for one’s self and others that contribute to the individual’s longer-term welfare. In a sense, it is the effective use of self-regulation in making choices in one’s life. When low, Conscientiousness acts to reduce life expectancy due to its being a background trait that predisposes people to engage in adverse health and lifestyle activities that are known to reduce life expectancy, such as smoking, alcohol and drug use, risk taking, poor health maintenance activities, poor diet, etc. Indeed, low Conscientiousness has been repeatedly shown to mediate the risk of earlier death by all causes (Boff & Roberts, 2004). The trait is negatively related to self-regulation generally and behavioral disinhibition specifically. Naturally, then, it is also negatively related to ADHD that includes disinhibition as one of its central symptom dimensions. Hence, if those in the bottom quartile on this trait had a significant reduction in lifespan, even among gifted children, those with ADHD would be expected to have an even greater reduction in life expectancy as those with ADHD place in the bottom 5-7 percent of the population in their poor inhibition.
Besides being low in Conscientiousness, there are a number of other reasons to expect that ADHD would be linked to a reduced life expectancy by young adulthood. For one thing, ADHD is linked to increased adverse consequences in nearly every major domain of life activity studied to date, some of which are linked to shortened life expectancy. For instance, ADHD is associated with higher risks for accidental and self-inflicted injuries in childhood and adulthood. Adverse driving outcomes, including more vehicular crashes are also associated with ADHD. ADHD further increases the risk for suicidal ideation (along with comorbid depression), attempts (associated with impulsivity), and completions. And teens and adults with ADHD-C are far more likely to be involved in interpersonal hostility generally and antisocial activities specifically that include violent crimes, reactive aggression, and intimate partner violence even when conduct disorder is not present or is statistically controlled. All of these variables would predispose to an increased risk for greater morbidity and likely earlier mortality by violent means.
Another line of evidence also suggests that ADHD should be linked to shortened life expectancy. ADHD is associated with various adverse medical conditions. These include:
- Upper respiratory infections (39-44%), asthma (22% or 2x greater risk), otitis media, allergies such as rhinitis, and skin disorders like eczema or acne (2.5x greater risk) in children with ADHD.
- Nonspecific lung, cardiovascular, and other chronic diseases in adults with ADHD.
- Poorer dental hygiene and greater risk for diseased, missing, filed, or traumatically injured teeth, gum disease, and bruxism in children with ADHD. For instance, the risk for oral trauma alone is 5x greater than in typical children.
- Greater substance use, misuse, dependence, and abuse such that 20-30% of teens and adults with ADHD may qualify for a substance dependence or abuse disorder. ADHD has been specifically linked to excess use of alcohol by adulthood, tobacco use in adolescence and adulthood, and marijuana use by adulthood. Teens and adults with ADHD are 2-3 times more likely to use tobacco than typical peers. If comorbid with conduct or antisocial personality disorder, those with ADHD are also predisposed to abuse of cocaine, methamphetamine, heroin, or illegal use of prescription drugs.
- Obesity: Although not evident in studies done 30-50 years ago, within the past 10 years, numerous studies have found that teens and adults with ADHD are more likely to be overweight and 1.5-3x more likely to qualify as having obesity with several studies showing up to 41% as having obesity. Longitudinal studies find that this risk, while small in childhood, increases over development. This more recent evidence of obesity being linked to ADHD likely reflects the greater availability of junk food to more recent generations than was available in earlier decades.
- Eating pathology: Females with ADHD, especially in late adolescence, are 3.6 times more likely (than females without ADHD) to suffer from eating pathology, specifically impulsive eating and binge eating, and 5.6x more likely to have bulimia, such that 15-20% qualify for a diagnosis of an eating disorder.
- Enuresis (2-5x) and encopresis (5x) in children with ADHD than typically developing children.
- Seizure disorders (2.5-4x) in children with ADHD.
- Sleeping problems (52-64%) in ADHD as compared to typical children (21-27%), including insomnia, frequent night waking, noisy sleep and breathing difficulties while sleeping (such as snoring, sleep apnea or airflow obstruction), restless sleeping and even restless leg syndrome, inefficient sleep, early waking, and consequently greater daytime sleepiness and inattention. Though less well studied, several studies found adults with ADHD to be more likely to experience these same sorts of sleeping difficulties.
- Excessive internet use and addiction, and gaming addiction (34%).
- Exposure to traumatic events and victimization, with figures as high as 62% for children with ADHD and 91% for those with comorbid oppositional defiant disorder, with rates of physical abuse estimated to be 3x greater (14.3% vs. 4.5% of typical children).
- Risky sexual behavior, earlier age at first sexual intercourse, greater number of sex partners, 4x risk for sexually transmitted disease, and 8-10x risk for teenage pregnancy.
- Poorer nutrition, greater consumption of sugars and carbohydrates.
- Greater sedentary lifestyle, less physical exercise.
- Type II diabetes: As a correlate of their greater rates of having obesity, poorer diets, and limited exercise, teens and adults with ADHD are 2.8-3.3x more likely to develop Type 2 Diabetes.
- Coronary heart disease: As a result of the factors cited just above along with their greater penchant for substance use and abuse, some evidence now suggests that those with ADHD may also manifest a significantly greater risk for future coronary heart disease as early as age 27, and double the risk for dementia in later life.
Although significant health adversities in their own right, many of these conditions are well-known correlates of reduced life expectancy and are used in algorithms that predict life expectancy as occurs in public health research and in the life insurance industry. Demontis et al. (2018) reported the results of a meta-analysis of DNA from 35,191 control cases and 20,183 of ADHD cases. It is the first genome-wide meta-analysis of ADHD risk gene loci to have found at least 12 chromosomal sites or loci to be significantly linked to ADHD. But the finding most relevant to this discussion was that of significant shared genetic relationships between ADHD and nearly all of the first order risk factors used in our computation of ELE, including educational attainment, obesity, diabetes, smoking, sleep, etc. This also applied to other factors we did not use, such as level of high density lipid cholesterol, earlier age of parenthood, risk for rheumatoid arthritis, earlier menopause, etc. It also found a genetic correlation between ADHD and lower intelligence, which we also found to be significantly predictive of variation in ELE. They also reported a significant and negative shared genetic relationship between ADHD and earlier parental mortality in both mothers and fathers. This further supports our conclusions that ADHD and its linkage to poor inhibition and low Conscientiousness are important genetic background or second order factors that link to the first order factors that are shortening the life expectancy in those with ADHD.
ADHD and Early Mortality
Over the past decade, some research has specifically examined the issue of greater mortality in ADHD using large epidemiological samples or even entire populations. That body of evidence showed the following:
- In 2012, Rachel Klein, Salvatore Mannuza, and colleagues, who have conducted the longest follow-up study of ADHD children into midlife, found that twice as many had died by age 41 as in their control group (7.2% vs. 2.8%). But the sample sizes in such studies of clinic-referred children are inadequate to detect such differences as being significant with any degree of statistical power.
- Even so, such findings have now been confirmed in multiple epidemiological studies of populations in the United States (Jokela, Ferrie, & Kivimaki, 2009; London & Landes, 2016), Denmark (Dalsgaard, Ostergaard, Leckman, Mortensen, & Pedersen, 2015), Sweden (Sun et al., 2019; Virtanen et al., 2018), Taiwan (Chen et al., 2019), and the United Kingdom (O’Nions et al., 2025). These studies show that
- children with ADHD are nearly twice as likely to die in childhood and
- adults with ADHD are 3 to nearly 5 times more likely to die in adulthood by midlife compared to typical people.
- Indeed, during any 4-year period, adults with ADHD in the US are almost twice as likely to die than typical adults.
The greatest cause of early mortality in these studies is accidental injury. But death by suicide is also a significant factor although in a far lower percentage of cases. And the Taiwanese study found that death from homicide was twice as likely than in typical peers.
- This risk of dying younger than usual was also found in the genome-wide study of ADHD genetics and its relationship to medical and psychological factors in affected patients and families where the parents of people with ADHD also were more likely to experience earlier mortality (Demontis et al., 2018).
ADHD and Estimated Life Expectancy
These studies do not address, however, the cumulative risk of chronically engaging in adverse health and lifestyle activities, such as those enumerated above, that can reduce life expectancy after mid-life. Thus, Mariellen Fischer, Ph.D. and I decided to use our Milwaukee longitudinal study to examine the possibility of reduced life expectancy in our ADHD and control children at their young adult follow-up (mean age 27 years) by entering 14 variables related to disability, health, and lifestyle into a recently available estimated life expectancy (ELE) calculator provided by the University of Connecticut Goldenson Center for Actuarial Research. We then proceeded to undertake a more complete analysis of our data the results of which were the first estimates ever reported on life expectancy of children with ADHD by young adulthood (see Barkley & Fischer, 2019). The initial findings were used to create the graphs shown below (SEs = standard errors). The first figure compares the ELE for children with and without a childhood diagnosis of ADHD Combined presentation (ADHD-C; then called Hyperactive Child Syndrome).
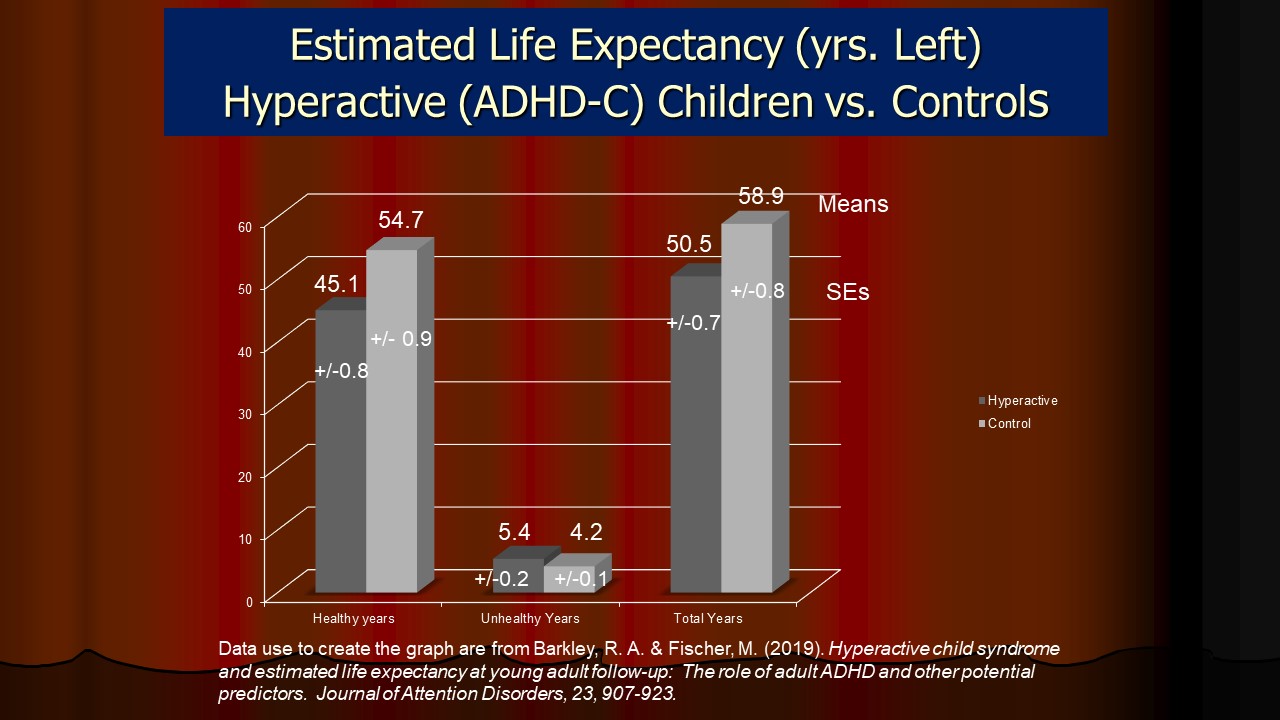
Here one can see that cases having ADHD-C in childhood manifested a 9.6 year reduction in estimated healthy life expectancy in remaining years, a 1.2 year period of greater unhealthy life expectancy in remaining years, and an overall 8.4 year reduction in total life expectancy than did control children by young adulthood. Thus, regardless of subsequent persistence of disorder, treatments and life course, having ADHD in childhood is associated with a substantial reduction in ELE, both healthy years remaining and total life remaining. But what was the impact on ELE if ADHD was found to persist to young adulthood? Those findings are shown in the next Figure.
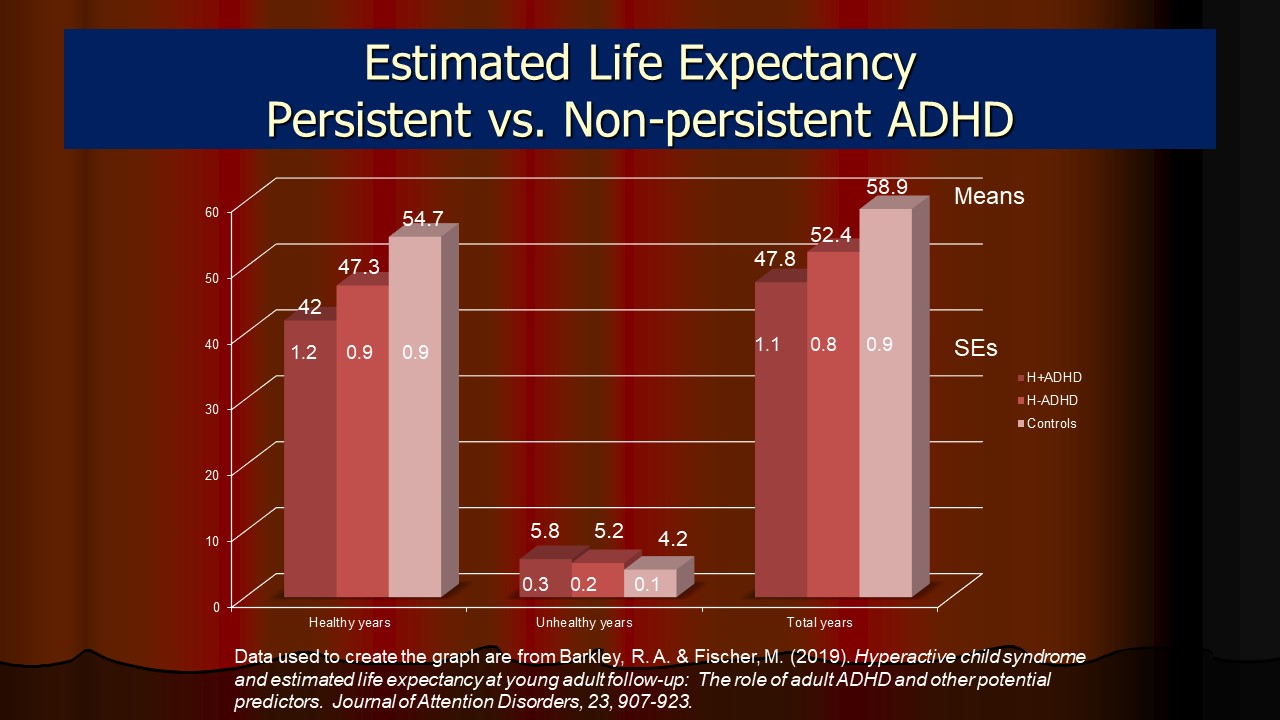
As is evident there, the persistence of ADHD to adult follow-up was associated with an even worse impact on these ELE measures, with a 12.7-year reduction in healthy life expectancy and an 11.1-year reduction in total ELE than was seen in control cases. Persistent cases had a 5.3-year reduction in healthy life expectancy and a 4.6-year reduction in total ELE than nonpersistent ADHD-C cases. And both persistent and nonpersistent ADHD cases had significantly lower ELEs by adulthood than did control cases. More recently, O’Nions et al. (2025) used a large population sample in the United Kingdom and found life expectancy to be reduced by 6-7 years in males and 8-9 years in females with the disorder compared to same sex control cases.
The magnitude of such reductions in life expectancy can be appreciated by understanding that such reductions are far greater than those associated with smoking, obesity, alcohol use, high cholesterol, and high blood pressure either individually or combined! For instance, obesity is associated with a -4.2 year reduction in life expectancy (Fekri et al., 2019), or -7 months per unit of BMI overweight (Joshi, Pirastu, Kentistou, Fischer, Hofer et al., 2017), smoking 20+ cigarettes per day with approximately -6.8 years (Joshi et al., 2017), excessive alcohol use with -2 years in men and -0.4 years in women (Makela, 1998), substance use disorder with 10 years (Chesney et al., 2014), elevated blood pressure with -5.2 years, and for every year of education after high school was +11 months (Joshi et al., 2017). Thus, ADHD has a more adverse effect on life expectancy than any single adverse health event noted above, and on which insurers, governments, and individuals spend billions of dollars to reduce those risks.
Why? Because ADHD has been found to predispose individuals to engage in a number of such adverse health and lifestyle activities. For instance, we noted that the disorder reduced ELE in our study through its association with 8 of the 14 variables entered in the ELE calculations. These included the demographic factors of reduced education, lack of high school graduation, and lower annual income in the ADHD-C groups but also in the health and lifestyle factors of greater alcohol consumption, poorer overall health, reduced sleep, increased likelihood of smoking and of smoking more than 20+ cigarettes per day, and possibly greater adverse driving consequences resulting in license suspensions and revocations. Beyond these first order or more proximal factors that were adversely affecting life expectancy, we showed that the background trait of behavioral disinhibition explained more than 30% of the variance in life expectancy in our samples, consistent with the findings noted above concerning the role of low Conscientiousness in reducing lifespan.
This finding concerning the impact of ADHD on shortening life expectancy is quite consistent with research on life expectancy associated with a failure to engage in at least 5 well-known health improvement practices. Li and colleagues (2019) used the ongoing longitudinal study of 120,000 people in the Nurses’ Health Study and divided them into those who engaged in five well-known health maintenance practices or not. These practices were: (1) nonsmoking, (2) moderate exercise (30+ min. per day), (3) maintaining a body mass index below 25, (4) engaging in moderate alcohol intake, and (5) adopting a high quality diet (low in fats, red meat, carbs, and sugar). Those who engaged in all five practices had a life expectancy 12-14 years greater than those who did not. As shown above, people with ADHD are far less likely to engage in these health maintenance practices and thus would be expected to have this much reduction in their life expectancy - precisely what the Barkley and Fischer study found.
Just as important was the finding by Barkley and Fischer that, beyond these first order or more proximal factors that were adversely affecting life expectancy, the background trait of behavioral disinhibition (linked to greater ADHD symptoms or low conscientiousness) explained more than 30% of the variance in life expectancy in their samples, consistent with the findings noted previously by many others concerning the role of low conscientiousness in reducing lifespan (Hampson, 2008). Behavioral inhibition is one of the major executive functions that is routinely deficient in ADHD. In other words, it is ADHD and its underlying executive function deficits [especially its dimension of high impulsivity or disinhibition] that is accounting for the predisposition to routinely engage in these various adverse health related activities and so to predispose toward a shorter life expectancy. Failing to appreciate this substantial second order background factor of ADHD and particularly related disinhibition could easily lead to both (a) overlooking its role not only in contributing to poor health-related behavior but also (b) understanding why certain individuals may have such limited success with adhering to recommendations for health improvement activities intended to lessen those first order risk factors. Thus, professionals must identify and address the second order background risk being posed by ADHD and especially behavioral disinhibition when dealing with patients who experience the first order health risks.
The evidence to date is more than sufficient to justify alarm at the substantial adverse impact ADHD poses for long-term quality of life and reduced life expectancy, as well as the call to action posed in this white paper report. But that call to action is made all the more compelling when one considers the economic costs and burden that ADHD poses to affected individuals, their families, employers, insurers, government public health agencies, and society at large.
These findings in the context of the other research noted above on increased mortality by mid-life argue for ADHD being viewed as a public health and not just a mental health disorder. They should also give impetus to efforts to try to reduce those first order factors that are predisposing to reduced life expectancy, such as obesity, smoking, excess alcohol use, poor diet, poor sleep, limited exercise, etc. in children and adults with ADHD. After all, estimated life expectancy is malleable – change the adverse health and lifestyle factors affecting it and one can improve quality of life as well as life expectancy. But our results also suggest that without efforts to address the background trait of poor inhibition specifically and ADHD symptoms more generally, trying to improve only those first order factors may have only limited success. Adding ADHD medications will likely prove helpful to doing so. We now know this in view of multiple studies that show a reduction in many of these domains of adverse events as a consequence of medication treatment for ADHD (see meta-analysis by Boland et al., 2020). Adding other evidence based psychosocial treatments to address the background traits predisposing those with ADHD to engage in these first order adverse activities, as well as targeting the first order risk events directly, is also likely to be useful. These findings also argue for making primary care physicians more aware of the linkage between ADHD, poor inhibition, and reduced life expectancy as they are the one’s most likely to be trying to improve the adverse health and lifestyle activities of individuals and yet are not screening for the significant role that ADHD may be playing in their failures to do so. In summary, ADHD predisposes to numerous adverse outcomes across a wide variety of domains of major life activities, as shown in the Figure below. Thus, it is hardly a benign disorder and one that now should be considered as a public health disorder, not just a mental health disorder.
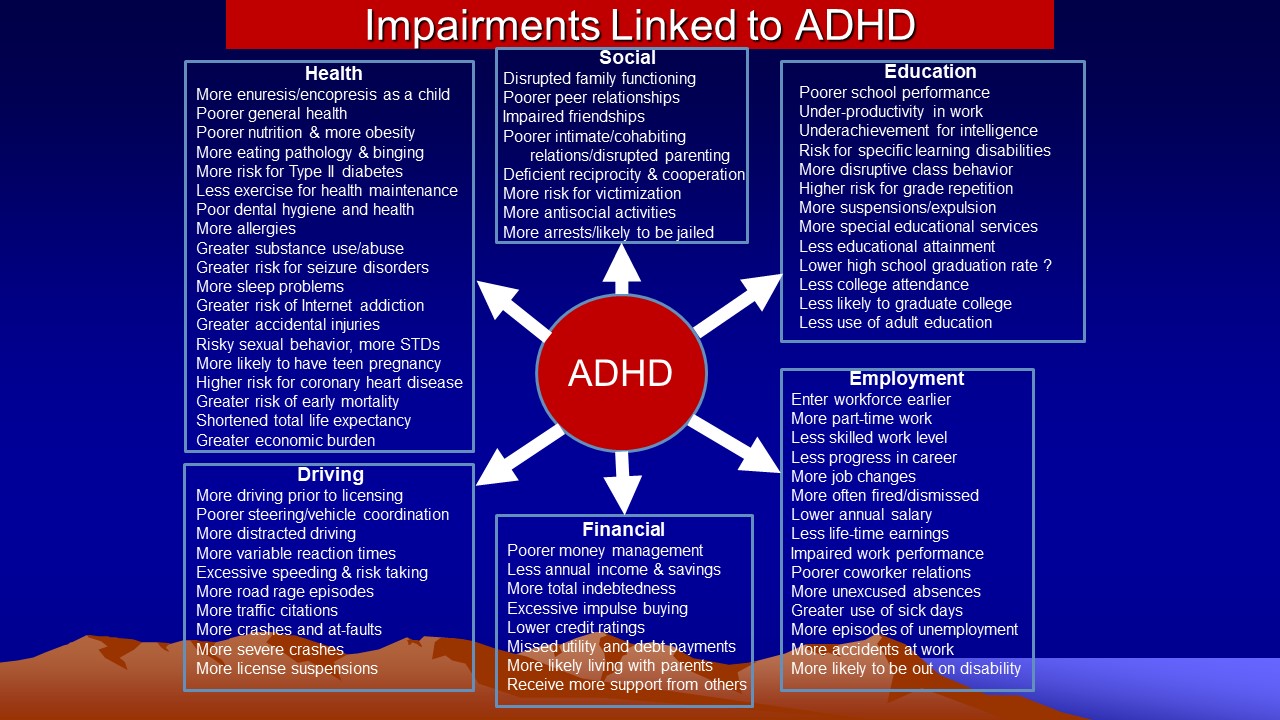
ETIOLOGIES
Considerable research has accumulated on various etiologies for ADHD. Notably, virtually all of this research pertains to the DSM-IV Combined Type of ADHD or what was previously considered hyperactivity in children. Readers should not extend these findings to the other attention disorder noted above to have sluggish cognitive tempo, which is likely a qualitatively different disorder. But for ADHD, there is even less doubt now among career investigators in this field that, while multiple etiologies may lead to ADHD, neurological and genetic factors likely play the greatest role in causing this disorder. These two areas, along with the associated field of the neuropsychology of ADHD, have witnessed enormous growth in the past decade, further refining our understanding of the neuro-genetic basis of the disorder. Our knowledge of the final common neurological pathway through which these causes produce their effects on behavior has become clearer from converging lines of evidence employing a wide array of assessment tools, including neuropsychological tests sensitive to frontal lobe functioning, electrophysiological measures (EEG, QEEG, ERP), measures of cerebral blood flow, and neuro-imaging studies using positron emission tomography (PET), magnetic resonance imaging (MRI), and functional MRI.
Several recent studies have even identified specific protein abnormalities in specific brain regions that may be linked to possible neurochemical dysregulation in the disorder. Precise neurochemical abnormalities that may underlie this disorder have proven extremely difficult to document with any certainty over the past decade, but advancing psychopharmacological, neurological, as well as genetic evidence suggests involvement in at least two systems, these being dopaminergic and noradrenergic. Neurological evidence has converged on the brain networks involved in ADHD, as discussed below. Whereas most early findings on etiologies were correlational in nature, not permitting direct, precise, immediate molecular evidence of primary causality, more recent evidence in this past decade has provided the confirmatory evidence of precisely which brain regions and their networks are creating the symptoms of ADHD.
Neurological Factors
A variety of neurological etiologies have been proposed for ADHD. Brain damage was initially proposed as an initial and chief cause of ADHD symptoms (Still, 1902), either occurring as a result of known brain infections, trauma, or other injuries or complications occurring during pregnancy or at the time of delivery. Several studies show that brain damage, particularly hypoxic/anoxic types of insults, is associated with greater attention deficits and hyperactivity. ADHD symptoms also occur more in children with seizure disorders that are clearly related to underlying neurological malfunction. However, most ADHD children have no history of significant brain injuries or seizure disorders, and so brain damage is unlikely to account for the majority of children with ADHD.
Throughout the century, investigators have repeatedly noted the similarities between symptoms of ADHD and those produced by lesions or injuries to the frontal lobes more generally and the prefrontal cortex specifically. Both children and adults suffering injuries to the prefrontal region demonstrate deficits in sustained attention, inhibition, regulation of emotion and motivation, and the capacity to organize behavior across time.
Neuropsychological Studies
Much of the neuropsychological evidence pertaining to ADHD was reviewed above under the particular forms of cognitive impairment seen in ADHD. A large number of studies have used neuropsychological tests of frontal lobe functions and have detected deficits on these tests, albeit inconsistently. Results suggest that it is poor inhibition of behavioral responses, or what Nigg (2001, 2006) has called executive inhibition, that is solidly established as impaired in this disorder. As noted earlier, evidence also exists for related difficulties as well, including with nonverbal and verbal working memory, planning, verbal fluency, response perseveration, motor sequencing, sense of time, and other frontal lobe functions. Adults with ADHD have also been shown to display similar deficits on neuropsychological tests of executive functions. One recent study of adults also found diminished olfactory identification in adults with ADHD, a finding predicted on the basis that both executive functions and olfactory identification are mediated by prefrontal regions. Moreover, research shows that not only do siblings of ADHD children who have ADHD show similar executive function deficits, but even those siblings of ADHD who do not actually manifest ADHD appear to have milder yet significant impairments in these same executive functions. Such findings imply a possible genetically linked risk for executive function deficits in families having ADHD children, even if symptoms of ADHD are not fully manifest in those family members. Supporting this implication is evidence that the executive deficits in ADHD arise from the same substantial shared genetic liability as do the ADHD symptoms themselves and as does the overlap of ADHD with ODD/CD.
Important in recent studies in this area has been the demonstration that these inhibitory and executive deficits are not the result of comorbid disorders, such as ODD, CD, anxiety, or depression, thus giving greater confidence to their affiliation with ADHD itself. This is not to say that some other disorders, such as learning disabilities or autism, do not affect some executive functions, such as those of verbal working memory, perhaps owing to their associated deficits in language development, but that the pattern of deficits associated with ADHD is not typical of these other disorders. The totality of findings in the neuropsychology of ADHD is impressive in further suggesting that some dysfunction of the prefrontal lobes (inhibition and executive function deficits) is involved in this disorder. Despite such findings, only 35% to 50% of children or adults with ADHD are likely to fall in the impaired range on various tests of EF but this rises to 86% to 98% of cases if rating scales of EF are employed. Since the ratings have far greater ecological validity than do the tests of EF, such evidence supports the assertion that ADHD is in fact a disorder of EF.
Neurological Studies
Early research in the 1960s and '70s focused on psychophysiological measures of nervous system (central and autonomic) electrical activity, variously measured (electroencephalograms, galvanic skin responses, heart rate deceleration, etc.). These studies were inconsistent in demonstrating group differences between ADHD and control children in resting arousal. But where differences from normal are found, they are consistently in the direction of diminished reactivity to stimulation, or arousability, in those with ADHD. Research continues to demonstrate differences in skin conductance and heart rate parameters in response to stimulation in those with ADHD that may distinguish them from children with conduct disorder or those with comorbid ADHD/CD.
Far more consistent have been the results of quantitative electroencephalograph (QEEG) and evoked response potential (ERP) measures, sometimes taken in conjunction with vigilance tests. Although results have varied substantially across these studies, the most consistent pattern for EEG research is increased slow wave, or theta, activity, particularly in the frontal lobe, and excess beta activity, all indicative of a pattern of under-arousal and under-reactivity in ADHD. ADHD children have been found to have smaller amplitudes in the late positive and negative components of their evoked response patterns (ERPs). These late components are believed to be a function of the prefrontal regions of the brain, are related to poorer performances on inhibition and vigilance tests, and are corrected by stimulant medication. Thus, psychophysiological abnormalities related to sustained attention and inhibition indicate an under-responsiveness of ADHD children to stimulation that is corrected by stimulant medication.
Several studies have also examined cerebral blood flow using single photon emission computed tomography (SPECT) in ADHD and normal children. They have consistently shown decreased blood flow to the prefrontal regions (most recently in the right frontal area) and pathways connecting these regions to the limbic system via the striatum, and specifically its anterior region known as the caudate, and with the cerebellum. Degree of blood flow in the right frontal region has been correlated with behavioral severity of the disorder, while that in more posterior regions and the cerebellum seems related to degree of motor impairment.
Studies using positron emission tomography (PET) to assess cerebral glucose metabolism have found diminished metabolism in adults, particularly in the frontal region, and in adolescent females with ADHD, but have proven negative in adolescent males with ADHD. An attempt to replicate the finding with adolescent females having ADHD in younger female ADHD children failed to find such diminished metabolism. Such studies are plagued by their exceptionally small sample sizes that result in very low power to detect group differences, and considerable unreliability in replicating previous findings. However, significant correlations have been noted between diminished metabolic activity in the anterior frontal region and severity of ADHD symptoms in adolescents with ADHD. Also, using a radioactive tracer that indicates dopamine activity, Ernst and colleagues were able to show abnormal dopamine activity in the right midbrain region of ADHD children and that severity of symptoms was correlated with the degree of this abnormality. These demonstrations of an association between the metabolic activity of certain brain regions and symptoms of ADHD and associated executive deficits is critical to proving a connection between the findings pertaining to brain activation and the behavior comprising ADHD.
Other neuro-imaging technologies offer a more fine-grained analysis of
brain structures using the higher resolution magnetic resonance imaging (MRI)
devices. Studies employing this technology find differences
in selected brain regions in those with ADHD relative to control groups.
To date, at least five brain regions have been implicated in producing ADHD. These are the dorsolateral and orbital frontal regions, mainly on the right side; the anterior cingulated cortex; the basal ganglia, especially the striatum; the cerebellum, principally the vermis on the right side; and the anterior portion of the corpus callosum (the splenium). The various brain regions often implicated in ADHD in the most recent MRI research
are illustrated in the Figure below. Here the right hemisphere of the brain
is shown, but the left hemisphere has been cut away to expose the location of
the striatum in relation to the prefrontal regions controlling movement specifically
and behavior generally.
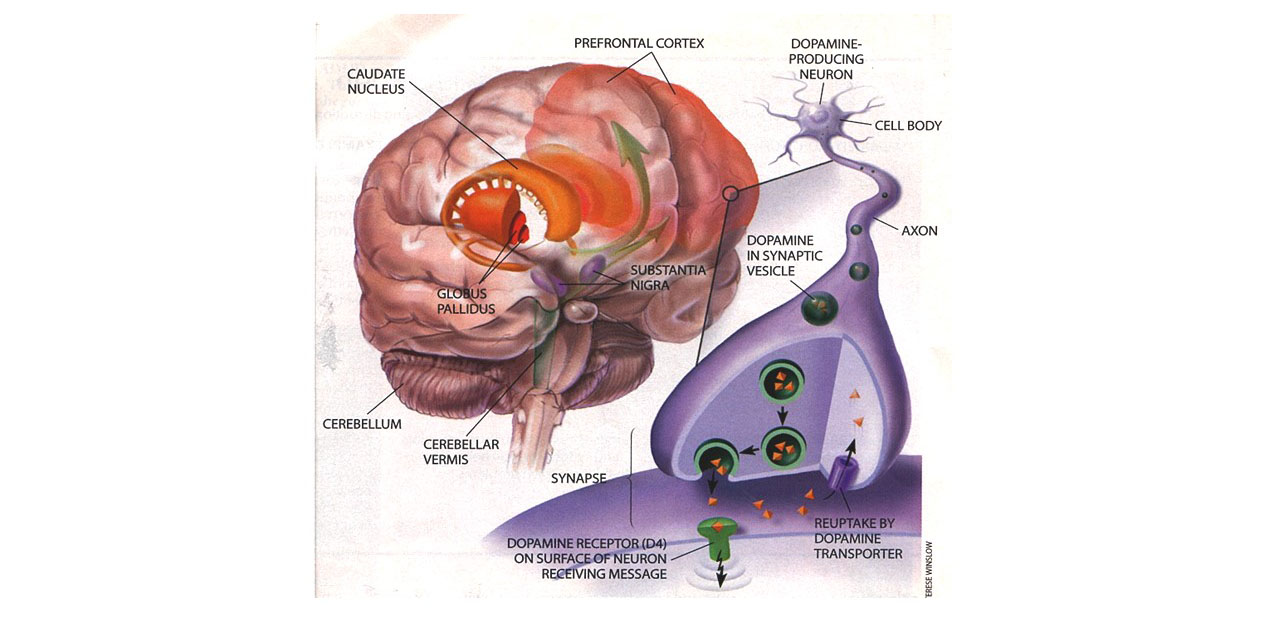
Diagram of the human brain showing the right hemisphere, and particularly the location of the caudate nucleus (striatum), globus pallidus, and cerebellar vermis. From R. A. Barkley (1998). Attention Deficit Hyperactivity Disorder. Scientific American, September, p. 47. Reprinted with permission of the artist, Terese Winslow.
With the advent of even more advanced functional MRI technology, researchers can now evaluate
functional activity in various brain regions while administering psychological
tests to subjects being scanned. These studies find children and adults with ADHD to have
abnormal patterns of activation during attention and inhibition tasks compared to
normal children, particularly in the right prefrontal region, basal ganglia
(striatum and putamen), anterior cingulated, and the cerebellum. Again,
the demonstrated linkage of brain structure and function with psychological
measures of ADHD symptoms and executive deficits is exceptionally important
in such research to permit causal inferences to be made about the role of these
brain abnormalities in the cognitive and behavioral abnormalities comprising
ADHD.
Neurotransmitter Deficiencies
Possible neurotransmitter dysfunction or imbalances have been proposed in ADHD for quite some time. Initially, these rested chiefly on the responses of ADHD children to differing drugs. ADHD children respond remarkably well to stimulants, most of which act by increasing the availability of dopamine via various mechanisms, and by producing some effects on the noradrenergic pathways as well. Consequently, it seemed sensible to hypothesize that these two neurotransmitters might be involved in the disorder. Given the findings that normal children show a positive, albeit lesser, response to stimulants, however, partially undermines this logic. Other, more direct evidence comes from studies of cerebral spinal fluid in ADHD and normal children that indicated decreased brain dopamine in ADHD children. Similarly, other studies used blood and urinary metabolites of brain neurotransmitters to infer deficiencies in ADHD, largely related to dopamine regulation. Early studies of this sort proved conflicting in their results. A subsequent study continued to find support for reduced noradrenergic activity in ADHD as inferred from significantly lower levels of a metabolite of this neurotransmitter. What limited evidence there is from this literature seems to point to a selective deficiency in the availability of both dopamine and norepinephrine, but this evidence cannot be considered conclusive at this time. Far greater evidence for involvement of these and other neurotransmitters comes from the rapidly growing evidence for the role of gene polymorphisms involved in regulating these neurotransmitters that reveals different gene variations in ADHD than in typical population samples.
Pregnancy and Birth Complications
Most studies have found a greater incidence of pregnancy or birth complications in ADHD compared to normal children. Children born prematurely or who have markedly lower birth weights are at high risk for later inattention, hyperactivity, or ADHD. It is not merely low birth weight that seems to pose the risk for symptoms of ADHD or the disorder itself, among other psychiatric disorders, but the extent of white matter abnormalities due to birth injuries, such as parenchymal lesions and/or ventricular enlargement. These findings suggest that some cases of ADHD may arise from such complications, especially from prematurity associated with minor bleeding in the brain.
Far more evidence exists for the role of exposure to various environmental toxins during pregnancy and a significantly elevated risk for ADHD, such as occurs in maternal smoking and alcohol consumption. And recent studies suggest that it is the interaction of these substances with certain high risk genotypes for ADHD that further elevate the risk for the disorder.
Genetic Factors
Evidence for a genetic basis to this disorder is now overwhelming and comes from four sources: family studies of the aggregation of the disorder among biological relatives, adoption studies, twin studies, and, most recently, molecular genetic studies identifying individual candidate genes.
Family Aggregation Studies
For years, researchers have noted the higher prevalence of psychopathology in the parents and other relatives of children with ADHD. Between 10% and 35% of the immediate family members of children with ADHD are also likely to have the disorder, with the risk to siblings of the ADHD children being approximately 32%. Even more striking, research shows that if a parent has ADHD, the risk to the offspring is 40% to 57%. Thus, ADHD clusters significantly among the biological relatives of children or adults with the disorder, strongly indicating a hereditary basis to this condition. Subsequently, these elevated rates of disorders also have been noted in African-American ADHD samples as well as in ADHD girls compared to boys.
These studies of families further suggest that ADHD with CD may be a distinct familial subtype of ADHD. By separating the group of ADHD children into those with and without conduct disorder (CD), it has been shown that conduct problems, substance abuse, and depression in the parents and other relatives are related more to the presence of CD in the ADHD children than to ADHD itself. Rates of hyperactivity or ADHD remain high even in relatives of the group of ADHD children without CD, but depression and antisocial spectrum disorders are most likely to appear in the comorbid group. Using sib-pairs in which both siblings had ADHD, Smalley and colleagues have also supported this view through findings that CD significantly clusters among the families of only those sib-pairs having CD.
Some research has also suggested that girls who manifest ADHD may need to have a greater genetic loading (higher family member prevalence) than do males with ADHD. Faraone and colleagues also found some evidence in support of this view in that male siblings from families with one affected child were more likely to have ADHD than were female siblings from these same families (Faraone et al., 1995). They also reported that the gender difference noted above for ADHD children (3:1 males-to-females) may apply primarily to children from families in which either the child or a parent has antisocial behavior.
Interestingly, research by Faraone and Biederman (1997) suggests that depression
among family members of children with ADHD may be a nonspecific expression of
the same genetic contribution that is related to ADHD. This is based on
their findings that family members of children with ADHD are at increased risk
for major depression while individuals having major depression have first-degree
relatives at increased risk for ADHD. Even so, as noted above, the risk
for depression among family members is largely among those children having ADHD
with CD.
Adoption Research
Another line of evidence for genetic involvement in ADHD has emerged from studies of adopted children. Early on, Cantwell (1975) and Morrison and Stewart (1973) both reported higher rates of hyperactivity in the biological parents of hyperactive children than in adoptive parents having such children. Both studies suggest that hyperactive children are more likely to resemble their biological parents than their adoptive parents in their levels of hyperactivity. Yet, both studies were retrospective and both failed to study the biological parents of the adopted hyperactive children as a comparison group. Cadoret and Stewart (1991) studied 283 male adoptees and found that if one of the biological parents had been judged delinquent or to have an adult criminal conviction, the adopted-away sons had a higher likelihood of having ADHD. A later study using biologically related and unrelated pairs of international adoptees identified a strong genetic component (47% of the variance) for the Attention Problems dimension of the Child Behavior Checklist, a rating scale commonly used in research on ADHD. More recently, a comparison of the families of adopted ADHD children to those living with their biological parents and to a control group also showed the same pattern of an elevated prevalence of ADHD among just the biological parents of the ADHD children (6% vs. 18% vs. 3% respectively). Thus, like the family association studies discussed earlier, results of adoption studies point to a strong possibility of a significant hereditary contribution to hyperactivity.
Twin Studies
The number of twin studies of ADHD and its underlying behavioral dimensions
is now substantial. There has been a striking consistency across all of
these studies in the pattern of their results. This research strategy
provides a third avenue of evidence for a genetic contribution to ADHD.
But it also provides a means of testing any competing environmental theories
of the disorder (e.g., that ADHD is due to poor parenting, adverse family life,
excessive TV viewing, etc.). That is because twin studies can not only
compute the proportion of variance in a trait that is genetically influenced
(heritability), but also the proportion that results from common or shared environment
(things twins and siblings have in common growing up in the same family) and
that which results from unique environment (all nongenetic factors or events
that are unique or specific to one child and not to others in the family).
Early research on ADHD using twins looked only at twin concordance (likelihood
of twins sharing the same disorder) and did not compute these estimates of heritability,
shared, and unique environment. These early studies demonstrated a greater
agreement (concordance) for symptoms of hyperactivity and inattention between
monozygotic (MZ) compared to dizygotic twins (DZ). Studies of very small samples of twins
found complete (100%) concordance
for MZ twins for hyperactivity and far less agreement for DZ twins. For
instance, Gilger, Pennington, and DeFries (1992) found that if one twin was
diagnosed as ADHD, the concordance for the disorder was 81% in MZ twins
and 29% in DZ twins. Sherman, McGue, and Iacono (1997) found that
the concordance for MZ twins having ADHD (mother-identified) was 67%
versus 0% for DZ twins.
Many subsequent studies have computed heritability and environmental contributions to ADHD. These twin studies have found a high degree of heritability for ADHD, ranging from.75 to.97 and averaging about.78. Thus, twin studies indicate that the average heritability of ADHD is approximately 0.73, being nearly that for human height (.80-.91) and higher than that found for intelligence (.55-.70). These studies consistently find little, if any, effect of shared (rearing) environment on the traits of ADHD, while sometimes finding a small significant contribution for unique environmental events. In their totality, shared environmental factors seem to account for 0% to 6% of individual differences in the behavioral trait(s) related to ADHD.
The twin studies cited above have also been able to indicate the extent to which individual differences in ADHD symptoms are the result of nonshared (unique) environmental factors. Such factors not only include those typically thought of as involving the social environment, but also all biological factors that are nongenetic in origin. Factors in the nonshared environment are those events or conditions that will have uniquely affected only one twin and not the other. Besides biological hazards or neurologically injurious events that may have befallen only one member of a twin pair, the nonshared environment also includes those differences in the manner in which parents may have treated each child. Parents do not interact with all of their children in an identical fashion and such unique parent-child interactions are believed to make more of a contribution to individual differences among siblings than do those factors about the home and child-rearing that are common to all children in the family. Twin studies to date have suggested that approximately 9% to 20% of the variance in hyperactive-impulsive-inattentive behavior or ADHD symptoms can be attributed to such nonshared environmental (nongenetic) factors. A portion of this variance, however, must be attributed to the error of the measure used to assess the symptoms. Research suggests that the nonshared environmental factors also contribute disproportionately more to individual differences in other forms of child psychopathology than do factors in the shared environment. Thus, if researchers were interested in identifying environmental contributors to ADHD, these studies suggest that such research should focus on those biological and social experiences that are specific and unique to the individual and are not part of the common environment to which the other siblings have been exposed.
Molecular Genetic Research
Most investigators now accept the fact that multiple genes contribute to the disorder, given the complexity of the traits underlying ADHD and their dimensional nature. At least 45 sites or more in the human genome have been implicated in the risk for ADHD based on reviews of genome-wide scans today. Among these, an abundant literature demonstrates a considerable role for the DAT1 (dopamine transporter) and DRD4 (dopamine receptor density) genes.
Environmental Toxins
As the twin and quantitative genetic studies have suggested, unique environmental events may play some role in individual differences in symptoms of ADHD. This should not be taken to mean only those social factors within the realm of psychosocial or family influences. As noted above, variance in the expression of ADHD that may be due to environmental sources comprises all nongenetic sources more generally. These include pre-, peri-, and post-natal complications; malnutrition; diseases; trauma; toxin exposure; and other neurologically compromising events that may occur during the development of the nervous system before and after birth. Evidence clearly suggests that most of these unique events or influences are likely to be biologically compromising events, several of which have been repeatedly linked to risks for inattention and hyperactive behavior.
One such factor is exposure to environmental toxins, specifically lead. Elevated body lead burden has been shown to have a small but consistent and statistically significant relationship to the symptoms comprising ADHD. However, even at relatively high levels of lead, less than 38% of children are rated as having the behavior of hyperactivity on a teacher rating scale implying that most lead poisoned children do not develop symptoms of ADHD. And most ADHD children, likewise, do not have significantly elevated lead burdens, although one study indicates their lead levels may be higher than in control subjects. Studies which have controlled for the presence of potentially confounding factors in this relationship have found the association between body lead (in blood or dentition) and symptoms of ADHD to be .10-.19 with the more factors controlled, the more likely the relationship falls below .10. Only 4% or less of the variance in the expression of these symptoms in children with elevated lead is explained by lead levels. Moreover, two serious methodological issues plague even the better conducted studies in this area: (1) None of the early studies used clinical criteria for a diagnosis of ADHD to determine precisely what percentage of lead-burdened children actually have the disorder – all simply used behavior ratings, comprising only a small number of items of inattention or hyperactivity; and (2) None of the early studies assessed for the presence of ADHD in the parents and controlled for its contribution to the relationship. Given the high heritability of ADHD, this factor alone could attenuate the already small correlation between lead and symptoms of ADHD by as much as a third to a half of its present levels.
Other types of environmental toxins found to have some relationship to inattention and hyperactivity are prenatal exposure to alcohol and tobacco smoke. It has also been shown that parents of children with ADHD do consume more alcohol and smoke more tobacco than control groups even when not pregnant. Thus, it is reasonable for research to continue to pursue the possibility that these environmental toxins may be causally related to ADHD. However, as in the lead studies discussed above, most of the early research in this area suffered from the same two serious methodological limitations – the failure to utilize clinical diagnostic criteria to determine rates of ADHD in exposed children, and the failure to evaluate and control for the presence of ADHD in the parents. More recent studies have addressed those concerns yet continue to show some relationships between these toxins and ADHD.
Psychosocial Factors
A few environmental theories of ADHD were proposed more than 30 years ago but have not received support in the available literature since then. Willis and Lovaas (1977) claimed that hyperactive behavior was the result of poor stimulus control by maternal commands and that this poor regulation of behavior arose from poor parental management of the children. Others have also conjectured that ADHD results from difficulties in the parents’ over-stimulating approach to caring for and managing the child, as well as parental psychological problems. But these conjectures have not articulated just how deficits in behavioral inhibition, executive functioning, and other cognitive deficits commonly associated with clinically diagnosed ADHD as described above could arise purely from such social factors. Nor is it at all obvious how such factors could lead to the substantial evidence now accumulated on the deficiencies in brain development and functioning associated with ADHD, not to mention the striking role of heredity and molecular genetics in the disorder that demonstrate ADHD to be among the most genetically influenced of all mental disorders. Moreover, many of these early studies proclaiming to have evidence of parental characteristics as potentially causative of ADHD have not used clinical diagnostic criteria to identify their children as ADHD, instead relying merely on elevated parental ratings of hyperactivity or laboratory demonstrations of distractibility to classify the children as ADHD. Nor have these purely social theories received much support in the available literature that has studied clinically diagnosed children with ADHD. The conclusion of the literature to date is that ADHD is likely to interfere with social relationships in both affected children and affected adults but that the disorder does not arise from such social relationships.
In view of the twin studies discussed above that show minimal, non-significant contributions of the common or shared environment to the expression of symptoms of ADHD, theories based entirely on social explanations of the origins of ADHD are difficult to take seriously any longer. This is not to say that the family and larger social environment do not matter, for they surely do. Despite the large role heredity seems to play in ADHD symptoms, they remain partly malleable to unique environmental influences and nonshared social learning. The types of comorbid disorders that will develop, the peer relationship problems that may arise, and various outcome domains impaired by the disorder are likely to be related in varying degrees to parent, family, and larger social factors. Yet even here, care must be taken in interpreting these findings as evidence of a purely social contribution to such conditions that may be associated with ADHD. This is because many measures of family functioning and adversity also show a strong heritable contribution to them, largely owing to the presence of similar symptoms and disorders (and genes!) in the parents as may be evident in the child. Thus, there is a genetic contribution to the family environment; a fact that often goes overlooked in studies of family and social factors involved in ADHD.
Summary
It should be evident from the research reviewed here that ADHD arises from multiple etiologies, neurological and genetic factors being substantial ones. Like Nigg (2006) and others, I envision ADHD as having a heterogeneous etiology with various developmental pathways leading to this behavioral syndrome.
This is illustrated in the figure below.
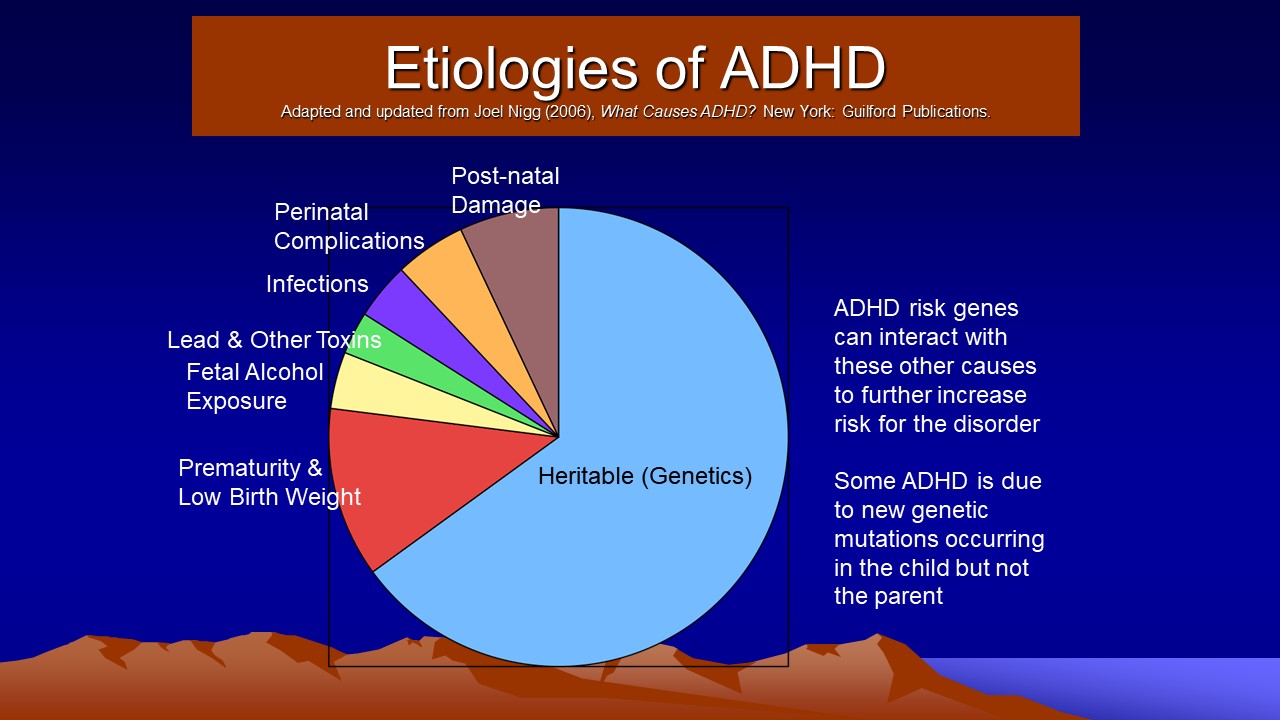
These various etiologies and pathways, however, may give rise to the disorder through disturbances in a final common pathway in the nervous system. That pathway appears to be the integrity of the prefrontal cortical-striatal-cerebellar networks. It now appears that genetic factors play the largest role in the occurrence of ADHD symptoms in children, reflecting both inherited risk genes for ADHD from parents as well as new mutations in these genes arising de novo in parental sperm and eggs and thus being transmitted to the child. It may be that what is transmitted genetically is a tendency toward smaller and less active or even defective prefrontal-striatal-cerebellar networks. The condition can also be caused or exacerbated by pregnancy complications, exposure to toxins, or neurological disease. Social factors alone cannot be supported as causal of this disorder but such factors may contribute to its persistence, and, more likely, contribute to the forms of comorbid disorders and the domains of impairment associated with ADHD. Cases of ADHD can also arise without a genetic predisposition to the disorder provided the child is exposed to significant disruption or neurological injury to this final common neurologic pathway, but this would seem to account for only a small minority of ADHD children. In general, then, research conducted since the last edition of this course has further strengthened the evidence for genetic and developmental neurological factors as likely to be causal of this disorder, while greatly reducing the support for purely social or environmental factors as having a role.
Cognitive Disengagement Syndrome (CDS) (Formerly Sluggish Cognitive Tempo (SCT) – A Second Attention Disorder Often Comorbid with ADHD
This section briefly reviews the evidence for the conclusion that there exists a second attention disorder, now termed cognitive disengagement syndrome (Becker et al., 2022). It is distinct from yet overlaps with attention-deficit/hyperactivity disorder (ADHD). The review is adapted from my chapter on this topic in the rating scale manual for evaluating SCT (Barkley, 2018) and supplemented with information from the recent review by Becker et al. (2022). The references supporting the findings discussed below are listed separately from those for ADHD at the very end of this course. The term SCT has been in use since it was first coined in the 1980s on research studying dimensions of attention problems in children with ADHD. Along with other researchers, I find the term “sluggish cognitive tempo” to be from ideal, and many are recommending it be changed to something less derogatory, pejorative, or frankly offensive. Also, the term is not accurate as SCT has been shown now to not be reliably associated with slow cognitive processing (Becker et al., 2022). A recent task force report recommended that the name be changed to Cognitive Disengagement Syndrome (CDS) for these reasons. I will refer to the condition by CDS throughout this discussion.
Some prior reviews have suggested that the condition be called attention deficit disorder, or ADD. Many clinicians have adopted this term for people who are primarily inattentive and have little or no evidence of hyperactive or impulsive behavior. While some of those cases likely involve CDS, I do not believe it is advisable to use the term ADD for this heterogeneous group. For one reason, ADD is the older term for ADHD dating back to the Diagnostic and Statistical Manual of Mental Disorders (3rd ed.), known as DSM-III (American Psychiatric Association, 1980). Resurrecting it as the name for a second attention disorder that is distinct in many ways from ADHD and from ADD, serves to create unnecessary confusion between the conditions. Moreover, ADD could be inferred to be a type of or presentation of ADHD, such as is the Primarily Inattentive Presentation now set forth in DSM-5-TR (American Psychiatric Association, 2022). As for continuing to use the term sluggish cognitive tempo CDS, the name clearly implies that the core cognitive deficit(s) underlying the condition has been definitively established (slow timing of thinking or information processing), which is not true. The same criticism can be applied to other terms suggested for something similar to this condition, such as Primary Disorder of Vigilance (PDV).
Cognitive disengagement syndrome, is an appropriate alternative label for for various reasons: (a) It has not been used for any other disorder previously; (b) It keeps the focus of the label on an attention problem yet makes it distinct from ADHD; (c) It is not offensive or pejorative to patients and family members as is SCT; (d) It does not imply we know more than we do about the underlying cognitive dysfunction, as do the terms SCT and PDV; and (e) It suggests some overlap with ADHD, which is in fact the case. Moreover, the term “cognitive disengagement” does not appear in the symptom lists for ADHD (DSM-5-TR) and thus is less likely to create unnecessary semantic confusion.
History of CDS vs. ADHD
Cases of CDS have likely existed within the human population at least throughout the past two centuries, if not longer. Descriptions of individuals with “low power” of attention or arousal, who appear to stare or daydream frequently, and to otherwise seem inattentive to or sluggish and erratic in accurately processing information, seem to first appear in the medical literature in Crichton’s medical textbook where he provided us with a description of two disorders of attention. One disorder clearly resembles what today we label as ADHD. In contrast to it, Crichton had little to say about the other disorder of attention except that it may be associated with debility or torpor of the body that weakens attention, thus causing individuals to be retiring, unsocial, and having few friendships or attachments of any kind. CDS may also have been the subject of a nursery rhyme written by the German psychiatrist, Heinrich Hoffman, entitled Johnny Head-in-the-Air, that was contained in his illustrated children’s storybook, Der Struwwelpeter, published in 1845. The book is often cited as an early reference to ADHD, as represented in the story about Fidgety Phil.
Apart from such historical curiosities, the contemporary period of research on CDS began in the 1980s and was clearly a consequence of the creation of two types of ADHD in DSM-III (American Psychiatric Association, 1980): those having an attention deficit disorder (ADD) with (+H) and without hyperactivity (-H). Studies soon began to appear that examined differences between children having each type of ADD. Their findings met with mixed success in differentiating them on features other than what would have been evident from their symptom requirements. That led to the removal of this subtyping approach in the subsequent DSM-III-R (American Psychiatric Association, 1987), only to see it return in the subtyping framework contained in DSM-IV as ADHD Combined Type vs. ADHD Predominantly Inattentive Type (American Psychiatric Association, 1994). This subtyping method was to be demoted again to the status of a mere “presentation” in the most recent DSM (DSM 5, American Psychiatric Association, 2022).
The specific identification of CDS seems to have begun in 1984 when Lahey and colleagues (1984) compared just 10 children with ADD+H to 20 children with ADD-H. They reported that the ADD+H group had significantly higher levels of aggressive behavior and conduct problems, bizarre behavior, lack of guilt, peer unpopularity, and poor performance in school. In comparison, the ADD-H children were more likely to be anxious, shy, socially withdrawn, and moderately unpopular, as well as poor in sports and impaired in school performance, all of which would later be replicated as correlates of CDS. The authors identified a set of symptoms in the ADD-H group that seemed to best characterize their attention problems, but were not part of the DSM symptom lists. Those symptoms comprised being drowsy, sluggish, and daydreamy, among others. In 1985, according to Carlson (1986), a student of Lahey’s (Neeper) conducted a cluster analysis in order to subtype learning-disabled children on the basis of their behavior using a rating scale that contained these and other items. He identified a separate group of 11 children having high scores on an inattention-disorganization factor and low scores in motor hyperactivity. That separate factor was then named a “sluggish tempo factor,” and comprised items related to apathetic, lethargic, sluggish, and drowsy behaviors. The condition has been referred to as sluggish cognitive tempo ever since.
In 2001, a very influential review of the literature concerning the two attention disorders (ADD+H and ADD-H) was published by Milich and colleagues (2001). The authors comprehensively reviewed research regarding comparisons of the subtypes. They also reviewed research using factor analysis of ratings of inattention. That research identified three distinct factors, two of which characterized ADHD, those being inattention and hyperactive-impulsive symptoms. CDS symptoms formed a third and distinct factor from these other two dimensions, and so the label CDS was applied to it. This was also found to be the case in a factor analysis of the direct observation form of the child behavior checklist by McConaughy and Achenbach (2001), where a separate attention deficit emerged independent of that typifying ADHD. Also in 2001, McBurnett, Pfiffner, and Frick conducted a factor analysis of ratings collected on 692 children referred to a specialty pediatric clinic for ADHD and found, as did Neeper earlier, that symptoms labeled as CDS formed a distinct dimension from the two traditional ones comprising ADHD. Three years later, Todd and colleagues (Todd, Rasmussen, Wood, Levy, & Hay, 2004) factor analyzed data from 2,894 twin pairs and also found a separate factor for CDS symptoms distinct from those for ADHD. Such a factor would emerge from all subsequent studies of the symptom lists of ADHD and CDS.
In the past decade, studies have focused specifically on children identified with high levels of CDS symptoms in comparison to those with ADHD. Some studies have estimated that as many as 30%-63% of cases of the Predominantly Inattentive cases of ADHD have high levels of CDS. Subsequently, many researchers have focused on groups of children, and later adults, with high levels of CDS symptoms. Indeed, Penny et al. went so far as to compile a comprehensive set of CDS symptoms according to experts they surveyed and a review of research papers. They then subjected the items to further analysis, ultimately creating a rating scale of the most useful set. By 2012, I had developed the first CDS rating scale for adults and published the results of the first study of adult CDS based on a representative U.S. sample of adults ages 18-92 (Barkley, 2012a), to be discussed further below. At the same time, I created a 12-item rating scale of CDS symptoms for children that was given to a representative sample of 1,922 U.S. parents to complete (Barkley, 2013) and that serves as the basis for this course and the BSCTS-CA.
In summation, the construct of CDS grew out of efforts to identify differences between subtypes of ADD and subsequently ADHD children. While differences between those subtypes proved mixed, weak, and unconvincing of any substantial or qualitative differences, research focusing specifically on children (and later, adults) having high levels of CDS symptoms proved much more promising in consistently identifying a variety of distinctions from ADHD. By 2016, enough research on CDS existed to conduct a meta-analysis of the existing literature that would conclude that on a number of variables, CDS seemed to be distinct from ADHD and worthy of much further study. Even so, in comparison to ADHD, the condition of CDS remains a highly under-studied problem with fewer than 70 articles currently existing on CDS in children and perhaps no more than 20 on adults. Increased demand for such empirically-based knowledge is likely to occur due to increasing clinical referrals of cases with this condition driven by increased awareness of the general public about CDS. The fact that CDS does not yet exist in any official taxonomy of psychiatric disorders does not alter this prediction. The increasing information on CDS at various widely visited Internet sites such as YouTube and Wikipedia, among others, will insure a growing public demand for more scientific knowledge about CDS and its management.
WHAT DO WE KNOW ABOUT THE NATURE OF CDS COMPARED TO ADHD?
The Best Symptoms for Identifying CDS
For a thorough review of what is known about CDS, see the review by Becker et al. (2022). While there is no official symptom list for CDS, as there is for ADHD, researchers have identified the most salient symptoms of CDS. My own research found the best symptoms for identifying CDS to be the following: (1) daydreaming; (2) trouble staying awake/alert; (3) mentally foggy/easily confused; (4) stares a lot; (5) spacey, mind is elsewhere; (6) lethargic; (7) under-active; (8) slow-moving/sluggish; (9) doesn’t process questions or explanations accurately; (10) drowsy/sleepy appearance; (11) apathetic/withdrawn; (12) lost in thoughts; (13) slow to complete tasks; and (14) lacks initiative/effort fades. The last two symptoms, however, were as likely to be associated with ADHD as with CDS in children and adolescents, so they are not recommended for assisting with differential diagnosis between these two types of attention disorders and do not appear on the Barkley Sluggish Cognitive Tempo Scale – Children and Adolescents (BSCTS-CA) contained in this course. But the remaining twelve, among others, are highly useful for identifying cases of CDS and so were used to form the BSCTS-CA in this course.
Recently, the meta-analysis by Becker and colleagues (2016) identified a set of 13 symptoms that seemed most useful to identifying CDS, primarily in children given that most such research in that analysis used that population. Subsequently, Becker and colleagues (2017) went on to develop a rating scale of CDS symptoms for adults, in which they identified 15 symptoms of CDS for use in their Adult Concentration Inventory (ACI). As is shown in Table 2 most of the symptoms from the meta-analysis are contained in their ACI scale. They then conducted a further validation study in adults and found that 10 of those symptoms on the ACI were most useful for identifying CDS in adults (distinguishing it from ADHD). Table 2, below, shows the results of these two efforts to identify the best candidate symptoms.
Table 2. Adult Concentration Inventory (ACI) Items and Support from Meta-Analysis and Validation in Adults |
ACI Items |
SCT Meta-Analysis |
Validation in Adults |
1. I am slow at doing things. |
✓ |
– |
| 2. My mind feels like it is in a fog. |
✓ |
– |
| 3. I stare off into space. |
✓ |
✓ |
| 4. I feel sleepy or drowsy during the day. |
✓ |
✓ |
| 5. I lose my train of thought. |
✓ |
✓ |
| 6. I am not very active. |
✓ |
– |
| 7. I get lost in my own thoughts. |
✓ |
✓ |
| 8. I get tired easily. |
✓ |
✓ |
| 9. I forget what I was going to say. |
– |
✓ |
| 10. I feel confused. |
✓ |
✓ |
| 11. I am not motivated to do things. |
✓ |
– |
| 12. I zone out or space out. |
✓ |
✓ |
| 13. My mind gets mixed up. |
– |
✓ |
| 14. My thinking seems slow or slowed down. |
✓ |
– |
| 15. I daydream. |
✓ |
✓ |
| 16. I have a hard time putting my thoughts
into words. |
– |
– |
| The meta-analysis is from Becker et al. (2016) and the adult validation study is from Becker et al. (2017). |
It should be noted that 10 of the 16 items identified in the meta-analysis above are contained on the BSCTS-CA form and 8 of the 10 items from the adult validation study likewise appear on this form. Note should be made here that symptom #11 was found in earlier research to be as much associated with ADHD as CDS and so was not a useful item, at least for children (see above). And symptoms 5, 7, 9, and 16 were found in factor analyses of the national survey reported later in this course to be far more strongly associated with a dimension of executive functioning (EF) in daily life related to Self-Organization (Barkley Deficits in Executive Functioning Scale – Child and Adolescents) and not with CDS. So 4 of the 16 items from the meta-analysis noted in Table 1.1 should not really be used to identify CDS as they will likely contaminate the construct with either ADHD (in the case of item 11) or EF (in the case of items 5, 7, 9, and 16). This is why they are not contained on the BSCTS-CA in this course.
More recently, Becker and colleagues (Becker, Burns, Schmitt, Epstein & Tamm, 2017) conducted a large study of 1,349 children in grades 2 through 5 using the following 16 items.
- Behavior is slow (e.g., sluggish) (0.92) ✔
- Lost in a fog (0.89) ✔
- Stares blankly into space (0.96) ✔
- Drowsy or sleepy (yawns) during the day (0.95) ✔
- Daydreams (0.88) ✔
- Loses train of thought (0.86) X
- Low level of activity (e.g., underactive) (0.97) ✔
- Gets lost in own thoughts (0.81) ✔
- Easily tired or fatigued (1.02) ✔
- Forgets what was going to say (0.94) X
- Easily confused (0.91) ✔
- Lacks motivation to complete tasks (e.g., apathetic) (0.27) X
- Spaces or zones out (0.82) ✔
- Gets mixed up (0.85) X
- Thinking is slow (0.87) ✔
- Difficulty expressing thoughts (e.g., gets “tongue-tied”) (0.78) X
In their exploratory and confirmatory factor analysis (contrasting these items with those of ADHD Inattention), they found that 15 of these 16 symptoms loaded highly on an CDS factor (factor loadings are in parentheses beside each symptom) and not significantly on the ADHD Inattention factor. Item #12 did not however; it was far more highly linked to the ADHD Inattention symptom dimension. As noted above, this again corroborates earlier research that this item dealing with motivation is not a good symptom for identifying CDS.
Of the remaining 15 symptoms, the authors found that item #14 (Gets mixed up) was highly correlated and thus redundant with item #11 (Easily confused) and so is unnecessary to include in a symptom list. And items #6, #10, and #16 dealing with problems organizing and expressing thoughts were found to be most highly associated with that dimension of executive functioning in daily life related to self-organization and not to CDS in the factor analysis to be reported later. The Becker, Burns, Schmitt et al. (2017) study did not use any measures of EF in daily life and so these three items appeared to load instead on their CDS dimension. As discussed below, prior research has shown that CDS has a small but significant association with this dimension of EF, so it is not surprising that in the absence of any EF measures, these items would seem to align with CDS. Yet given the opportunity to be analyzed alongside other EF symptoms, these apparent CDS symptoms then migrate over to the Self-organization dimension of EF and do not remain useful CDS items. For this reason, I again do not believe they should appear on a list of the best items for identifying CDS. And so I have placed an "X" next to each of those five symptoms to rule them out of further consideration.
With these issues in mind, that leaves just 11 of the 16 items on the above list as representing the best symptoms for CDS. And all 11 of these symptoms appear on the BSCTS-CA though not necessarily worded exactly as they appear here. I have placed a "✔" next to those items above that appear on the BCTS-CA. Also, two (#2 and #11) of the symptoms above are contained in a single item on the BSCTS-CA (Mentally foggy or easily confused). And the BSCTS-CA has an extra symptom reflecting difficulties remaining alert or awake that does not appear on the Becker et al. list above. Item 15 (Thinking is slow) above is represented on the BSCTS-CA as “Doesn’t seem to understand or process questions or explanations as quickly or accurately as others.” More on the phrasing and derivation of the CDS (SCT) symptoms on the BSCTS-CA will be presented later in this course, including the results of their factor analysis in comparison to ADHD and EF symptoms.
Thus, for now, it is safe to say that the BSCTS-CA utilizes the vast majority if not all of the best CDS symptoms identified in prior research as optimal for differentiating CDS from ADHD as well as from EF and other disorders even though it was initially developed prior to these two research reports by Becker and colleagues.
The Symptom Dimensions Are Distinct from ADHD
The symptoms of CDS seem to form either a single factor or at least two factors or dimensions in prior research. Those two dimensions identified are inter-correlated sufficiently to be combined in identifying this disorder. Most commonly, the two dimensions identified in factor analytic research are a Daydreamy/Spacey factor and a Sleepy/Sluggish/Underactive dimension or factor. In a study of adolescents with ADHD, a sleepy dimension emerged that was somewhat distinct from the Daydreamy and Sluggish factors but this is not the case in studies of children or when child and teenage samples are combined. In some studies, a separate factor is found for the low initiative and lack of persistence items (see #13 and #14 above). But as noted above these seem to be more related to ADHD Inattentive symptoms (IN) than to CDS (SCT) and thus would not be useful in differentiating CDS from ADHD. Interestingly, as with ADHD, there is a cognitive-inattentive dimension (Daydreamy/Spacey) and a motor dimension (Sluggish/Lethargic) to CDS, though they are clearly different from the symptoms comprising those general dimensions in ADHD. Smith and Langberg (2017) have argued that, at least in adolescents with ADHD, a multidimensional view of CDS as discussed above is preferable to a unidimensional view, given that the different dimensions appear to predict different forms of impairment (academic vs. internalizing problems).
The distinctiveness of CDS and its factors from those of ADHD have been evident across all of the various approaches to measurement studied to date, such as parent and teacher ratings, observations of behavior at school, and observations of behavior in clinical settings. CDS symptoms are not only separate from those of ADHD in children and youth but are also found to be separate from those for ADHD in adult self-reports for ages 18-89 and among college students.
Despite finding CDS symptoms to be distinct from those of ADHD across ages, measures and even sources, they may not necessarily correlate strongly across measurement sources. For instance, several studies have found only a low-to-moderate relationship between parent and teacher rated symptoms (rs = .29 - .38), showing that at most these ratings share just 14% of their variance. Although disappointing, such correlations are in keeping with the usual and modest levels of association found between parent and teacher ratings of all dimensions of child psychopathology.
CDS/SCT Symptoms Are Moderately Related to ADHD-Inattention, but Not Hyperactivity-Impulsivity
CDS symptoms have been found repeatedly to be significantly but moderately correlated with the ADHD Inattention symptom dimension, suggesting about 25%-36% shared variance between the two dimensions. Hence, they are not identical nor do they approach colinearity as if they derived from the same item pool or construct. Moreover, CDS symptoms identify a unique group of children even within samples that have ADHD Inattentive-Type. Yet CDS symptoms are substantially less correlated with ADHD symptoms than they are with each other. A number of studies find that CDS symptoms demonstrate a much weaker relationship to hyperactive-impulsive symptoms, if at all, than they do to IN symptoms. In fact, this relationship may become negative, at least for the CDS slowness dimension, once ADHD Inattention is statistically removed. All of this is to say that the structure of CDS symptoms is not merely a reflection or broadening of the ADHD Inattention symptom dimension. Instead, CDS symptoms are as independent or only partially coupled to ADHD symptoms as are other symptoms dimensions of child and adult psychopathology to each other.
CDS/SCT Symptoms Are Evident and Valid in Other Cultures/Countries
A small but growing body of evidence indicates that CDS symptoms can be identified in samples in other countries and cultures and that they show a similar pattern of distinctiveness from ADHD. Thus there is cross-cultural validity to this condition, at least as examined to date. Studies using samples of children from Canada, Spain, Korea, Nepal, and Chile, all attest to the existence and validity of CDS within these countries as a distinct condition from ADHD, so it is not strictly a U.S. phenomenon. This cross-cultural existence and validity is true of ADHD as well.
In sum, individual studies and meta-analyses of symptoms of CDS show that they are:
- coherent among themselves (more highly correlated with each other than with symptoms of other disorders, such as ADHD),
- form a distinct dimension or set of dimensions of symptoms from those comprising other disorders, such as ADHD, anxiety, and depression,
- are internally consistent (high internal reliability),
- show reasonable test-retest reliability over short time periods (Becker et al., 2016),
- have significant stability and invariance over long periods from 2 (Servera, Bernad, Carillo, Collado, & Burns, 2016) to 9 years (Leopold, Christopher, Burns, Becker, Olson, & Willcutt, 2016),
- show comparable low-to-moderate relationships between parent and teacher ratings as seen in other child psychopathologies,
- and are evident cross-culturally.
Demographic Differences between CDS and ADHD
Relationships to Age and Sex. Only a handful of prior studies examined parental/family demographic characteristics of CDS vs. ADHD. Several studies found that CDS was not related to child age, gender, or minority status. This same pattern was evident in my two large epidemiological studies of representative samples of U.S. children and adults across ages 6 to 89. And this was also the conclusion of the meta-analysis by Becker and colleagues (2016) where only a slight relationship of CDS symptoms to age was noted. In ADHD, however, the symptoms decline across childhood with age, especially those of hyperactivity-impulsivity. Groups having CDS have also been noted to be older than those having ADHD, implying a somewhat later age of onset for the former symptoms.
ADHD symptoms occur more often in boys than girls during childhood and adolescence but come close to equalizing in adulthood. This is not the case for CDS where males have only slightly more symptoms than females in childhood and no evident sex differences by adulthood. This lack of or very small association of CDS with age and sex was also evident in the recent study by Lee, et al. (2013). They noted no sex differences and no effect of age on teacher ratings and only a very small difference due to those demographic factors in parent ratings.
Some studies have found ADHD symptoms to be slightly but significantly associated with some ethnic groups (Hispanic-Latino) more than others, whereas this is not the case for CDS symptoms, either in large nationally representative samples, or in the meta-analysis by Becker and colleagues (2016).
Relations with Parental Factors. In my national survey of children, I noted that CDS was linked to lower parental education, lower annual household income, and a greater likelihood of a parent being out of work due to disability. A study in Spain also noted an association of this symptom dimension with paternal unemployment and low maternal education in a large sample of Catalan school children. My survey of U.S. adults also found that those classified as CDS also had less education and less annual income. In those instances where CDS was comorbid with ADHD in the adult survey, those cases were more likely to be unmarried and to be out of work on disability than were adults with ADHD. Such findings intimate that CDS might be more associated with psychosocial adversity or stressors than is ADHD. But the meta-analysis by Becker and colleagues (2016) concluded that CDS might be less related to such parental demographic features than is ADHD. To summarize, what patterns emerge in results to date indicate that the demographic correlates associated with CDS may be different from or far weaker than those evident in ADHD.
Differences in Cognitive Functioning
In general, there has been vastly less research on the neuropsychological deficits associated with CDS compared to ADHD, where the research literature is abundant. Studies are not consistent in showing a relationship of CDS to lower levels of intelligence. But the bulk of the evidence suggests a significant but modest negative relationship that may be most evident when teacher rated CDS is the source rather than parent ratings. I recently reviewed all of the research on neuropsychological studies of CDS so anyone interested in a detailed review of the topic should go there (Barkley, Willcutt & Jacobson, 2022).
A few studies imply that CDS may involve deficits in early information processing or selective attention that is not typical of ADHD. One recent study suggests that preschoolers with high CDS ratings may be more impaired on tests of visual-perceptual abilities, auditory and visual attention, sustained and selective attention, inhibitory control, and pre-numerical/numerical concepts even after controlling for ADHD inattention. Variability of spatial memory performance has also been specifically linked to CDS but not to ADHD in the Skirbekk, et al. (2011) study, even after controlling for IQ, ADHD inattention, and other variables. But such findings from so few studies must to be replicated in more research before being viewed as confirmed correlates of CDS.
Likewise, slower processing or motor speed has been linked to hypoactivity specifically, and to CDS symptoms more generally in some studies of children, consistent with its symptom profile. One recent study found that teacher rated CDS symptoms were significantly though modestly correlated with poorer performance on both a working memory task (WM) and the response parameter scores (mean reaction time, reaction time variability) of the attention network task (ANT), with rs = -.17 - .20 for WM, and .20 - .22 for ANT. But there was no association of CDS with those scores on the ANT linked to different attention networks (Alerting, Orienting, Conflict). And only the relationship of CDS to slow mean reaction time on the ANT remained significant after controlling for potential confounding variables, including ADHD inattention. In contrast, on reaction time tasks, ADHD is repeatedly associated with greater variability of reaction times.
One question raised by such findings is whether this problem of slow or sluggish speed in responding is the result of difficulties on the information processing side (perceptual speed) or the motor preparatory and execution side, or both. Current research has not been able to address this issue satisfactorily, yet the results of the Camprodon-Rosanas, et al. (2017) study imply it may be more of a motor slowing problem. And the problem of speed, wherever it arises, may not be so evident in older children and perhaps not at all by college age. Yet, as several studies found, even this association with slow speed in younger children is small and hardly convincing of it being the core deficit in CDS as its name clearly implies. Furthermore, others did not replicate this finding of slow perceptual-motor or processing speed despite studying children. Again, replication of such findings is essential before one can have confidence in their linkage to CDS. The meta-analysis of CDS research by Becker and colleagues (2016) likewise concluded that the neurocognitive research on CDS is too scant to draw any firm conclusions.
Only a few studies using psychometric tests of executive functioning (EF) specifically have been done with cases selected for CDS. Unlike ADHD, results intimate that CDS is not as serious and pervasive a disorder of EF, if it involves psychometrically assessed EF at all. In contrast, research is ubiquitous showing that in ADHD, for instance, there are deficits on tests of inhibition and working memory, especially nonverbal working memory. In contrast, this is not seen in CDS, where research shows that it is ADHD Inattention that is most closely linked to impaired EF test performance. When covaried out of analyses of CDS effects on EF, the result is often not significant. But the research here is so limited as to preclude any firm conclusions from being drawn. Moreover, EF tests have low or no ecological validity and low or no relationships to various domains of impairment in contrast to ratings of EF. And so EF tests cannot serve as the gold or sole standard for examining EF deficits in CDS (or any other disorder). Given their greater ecological validity and predictive power for impairment, EF ratings may provide a different pattern of results for CDS than do EF tests.
Just a few studies have used EF ratings to study cases of CDS. For instance, my own large studies used my rating scale of EF in daily life with large epidemiologically derived samples of children and adults having CDS, ADHD, or both. Results for the childhood survey showed that CDS had only very weak relationships to four of the five EF deficit dimensions (< 1% shared variance) when statistically controlling for its association with ADHD symptoms, especially the inattention dimension. On one dimension (Planning and Problem-Solving) there was a slightly higher contribution (< 5%) after such statistical control. A more recent study of adults with ADHD likewise found that CDS symptoms contributed primarily to the self-organization dimension of EF ratings but not to the other dimensions once ADHD symptoms were statistically controlled. A study of preschoolers similarly found that parent- and teacher-reported CDS symptoms were not related to ratings of EF in daily life. And as explained later in this course, when CDS symptoms are factor analyzed with ratings of EF in daily life, the two dimensions of CDS emerge as being distinct from those of EF. This is not the case for ADHD symptoms, which can be found cross-loading onto dimensions of EF rather than forming separate dimensions from them. Again, this implies that ADHD is very much a disorder of EF while CDS is not.
Several studies of college students did find some unique contribution of CDS symptoms to some dimensions of EF in daily life independent of ADHD Inattention but typically of a much smaller magnitude than is the case for ADHD Inattention. The only exception is in the self-regulation of emotion where Becker, et al. (2017) found CDS symptoms to make an equal contribution compared to the two dimensions of ADHD, whereas Jarrett, et al. (2017) found only CDS made such a contribution controlling for ADHD Inattention. This may be the case for adults with CDS symptoms, as was also found in my own U.S. survey of adults, but that was not the case in the survey of U.S. children. So, there may be some age-related changes in this relationship worth further study. Overall, it is the inattentive dimension of ADHD that contributes to the vast majority of variance across most EF dimensions on EF rating scales, with the hyperactive-impulsive dimension accounting for a lesser but still significant degree of variance, especially in the EF dimensions of Self-Restraint (inhibition) and Emotional Self-Regulation. From these results, it seems that CDS is not nearly as much a disorder of EF, if it is at all, as is ADHD, which is massively so on such ratings. Indeed, ADHD Inattention symptoms account for 8 to 20 times as much variance in most dimensions of EF ratings as do CDS symptoms.
Using a different rating scale of EF, Becker and Langberg (2013) also found a smaller contribution of CDS to the metacognitive factor on the Behavior Rating Inventory of Executive Functioning in comparison to the inattentive symptoms of ADHD. So did Jimenez and colleagues, even after controlling for ADHD inattention (Jimenez, Ballabriga, Martin, Arrufat, & Giacobo, 2013).
A small link of CDS to EF-like problems was also evident in a study by Langberg and colleagues (Langberg, Becker & Dvorsky, 2014) but only for parent-reported organizational problems. Yet only ADHD Inattention symptoms linked up with organizational problems as rated by teachers. It is possible that problems with certain aspects of working memory may be weakly related to, or possibly secondary to, the cognitive CDS daydreaming dimension. I believe those working memory/organizational problems hardly compare to the more severe and pervasive EF deficits so evident in ratings of daily life in children and adults with ADHD and which correlate so much more strongly with ADHD Inattention symptoms. Moreover, it is clear across all of these studies utilizing EF rating scales, that CDS has no significant association with EF inhibitory problems, whereas those inhibitory problems are substantial in ADHD.
Overlap of ADHD and CDS
The majority of research on CDS selected cases from among children referred to clinics for concerns about ADHD; indeed, in some, a diagnosis of some type of ADHD (via DSM-IV criteria) was the starting point. This can automatically make it seem as if CDS is highly associated with and hence a subtype of ADHD in the results of such research, if any differences emerge at all. It also means one cannot study the overlap or independence of the disorders, given the confounding starting point of identification of cases. But if CDS cases are selected specifically from general population or general outpatient clinic samples, there is the opportunity for CDS to be seen independently of ADHD and so the comorbidity between the two can be studied.
I did so in my two national surveys where I found that more than half (59%) of the children qualifying for a research diagnosis of CDS met research criteria for having ADHD. Markovich-Pilon et al. (2017) also found a high rate of ADHD (40%) in Canadian children referred to an ADHD clinic who were subsequently classified as having high levels of CDS symptoms. But this did not differ significantly from the rate (29%) found in those referred children rated low in CDS symptoms, perhaps due to low statistical power in the study design. A few studies suggest that it is among those ADHD subtypes having significant IN symptoms rather than with the Predominantly HI-Type that one is most likely to find the overlap with CDS. In my survey, only 39% of the children qualifying for ADHD of any type also qualified for CDS. Again, these findings agree with prior studies of children and adults (Barkley, 2012a). For instance, a recent survey of U.S. adults found that 5.8% of the sample met criteria for high CDS symptoms. Approximately half (54%) of those participants qualifying for CDS had ADHD, yet nearly half did not. Similarly, approximately half of individuals qualifying for ADHD of any type (46%) also qualified for CDS. It seems to me that the relationship of CDS to ADHD evident in these findings is one of co-morbidity between two relatively distinct but related or partially coupled disorders, such as exists between anxiety and depression, and not one of subtyping within a single shared disorder. More research will help clarify if this is, in fact, the case.
Patterns of Comorbidity
SCT symptoms are more often linked to elevated ratings of internalizing symptoms generally than are ADHD symptoms, especially the Slow dimension. This is so even after controlling for ADHD symptoms. Among adolescents with ADHD, self-report of CDS was more strongly predictive of internalizing symptoms than were parent reports, which better predicted academic impairment. This linkage of CDS to internalizing symptoms is undoubtedly one of the most reliable findings in the literature on CDS in child, teen, and adult samples. When the inverse is done such that CDS symptoms are statistically removed, the IN dimension of ADHD may be less related to, or even unrelated to, internalizing symptoms or even to ratings of social problems.
The relationships of CDS to anxiety and depression specifically are positive, not surprisingly given that the latter two conditions are often combined in ratings of internalizing symptoms. CDS may predict each of these internalizing dimensions (anxiety, depression) even after controlling for the overlap of the latter dimensions with each other. The association with depression may be stronger than that with anxiety and remains even after controlling for parental internalizing dimensions as was done by Becker, Luebbe, et al. (2013). While a few exceptions exist in this literature, the weight of the evidence finds CDS to be more closely related to internalizing symptoms (anxiety, depression, withdrawal) than is ADHD. There is a pattern here of a double dissociation between the two disorders in their linkage to internalizing symptoms that is evidence that they are distinct conditions from each other, not subtypes of a common disorder.
There is a lack of association of CDS with ODD. Furthermore, there is some more recent evidence that the relationships may be negative ones when ADHD Inattention is removed statistically in the analyses. So it can be reasoned that CDS also would have little or no associations with conduct disorder, substance use disorders, or adult antisocial personality disorder. That is because all of these risks are linked to varying degrees with ODD or the hyperactive-impulsive symptom dimension of ADHD, with which CDS is unassociated. Further evidence for this lack of association, or even negative association, with externalizing disorders is evident in a study using direct observations of disciplinary actions [time outs] received on an inpatient unit. Such disciplinary actions are often instituted for disruptive or aggressive behavior and were positively linked to the hyperactive-impulsive symptoms of ADHD while being negatively associated with CDS symptom severity. This is yet another double dissociation supporting the distinctiveness of CDS from ADHD.
One prior study examined the relationship of CDS vs. ADHD to specific professional diagnoses of 17 different learning, developmental, and psychiatric disorders as reported by parents concerning the past professional diagnoses their children had received. It found that both CDS and ADHD were associated with elevated rates of comorbidity for 11 of the 17 disorders. But CDS was not associated with higher rates of reading or math disorders, hearing impairment, ODD, anxiety, or bipolar disorder diagnoses than the controls. ADHD was linked to higher rates for all of these disorders except hearing impairments. Unlike ADHD, the CDS group had a higher rate of depression than either the controls or those with ADHD, consistent with other studies above finding such an association with ratings of depression. The comorbidity of ADHD+SCT was associated with higher rates of comorbidity for most disorders than was either disorder alone. This implies an additive effect of CDS and ADHD when they exist together as if each was a distinct disorder that rendered greater risks when comorbid. Or this pattern could have arisen merely as a function of symptom severity – comorbid cases had more symptoms of both disorders than was the case for each specific disorder group.
Even so, another study using Canadian children referred to an ADHD clinic did not find any differences in rates of comorbidity between the children with high vs. low CDS symptoms. This finding is in contrast to the repeated linkage of CDS/SCT with internalizing symptoms generally and depression specifically, noted above. Perhaps it results from a referral bias, however, in that clinic-referred children are more likely to have comorbid disorders than are non-referred children identified as disordered in epidemiological samples.
One recent study evaluated the extent to which CDS and ADHD symptoms were related to dimensions of psychopathy in a large sample (N = 198) of inpatient child mental health admissions. The two dimensions of psychopathy (psychopathy-impulsivity and narcissism) mediated the linkage between ADHD symptoms and social problems, whereas the symptoms of CDS were unrelated to psychopathy after controlling for their overlap with ADHD.
Domains of Impairment
For a condition to rise to the level of being a mental disorder, there must be evidence of impairment or harm (adverse consequences) to the individual from those symptoms (American Psychiatric Association, 2013). We can think of symptoms as the cognitive and behavioral expressions of a disorder, while impairment represents the consequences that flow from such symptoms.
Social Functioning. Studies of CDS symptoms have routinely shown it to be linked to social problems generally, and to social withdrawal specifically, even in the presence of high ADHD IN symptoms or after controlling for the overlap of ADHD IN symptoms with those of CDS. Such findings may be even more apparent in teacher than in parent ratings. This may be one of the most well documented associations of CDS with any domain of impairment. It differs markedly from the relationship of ADHD to social aggression and peer rejection.
Mikami and colleagues (Mikami, Huang-Pollack, Pfiffner, McBurnett, & Hangai, 2007) have provided the only study to date using detailed observations of the social interactions of children with CDS using a simulated chat room with children with ADHD and controls. They statistically controlled for ADHD type, IQ, reading ability, and typing skill in their analyses. CDS was noted to independently predict fewer total responses in the chat room, less perception of subtle social cues, less memory for the conversation, and a smaller proportion of hostile responses. While these findings agree with the more general findings above that CDS cases are more socially withdrawn, it also suggests a role of CDS in attention and an encoding dysfunction that accounts for impairment in critical social behaviors that are of a different sort than seen with ADHD (social intrusion, aggression, bossiness, excessive speech, etc.).
Noteworthy is that the association of CDS to social impairment or withdrawal remains even after statistically removing ADHD symptoms as well as those of ODD, CD, generalized anxiety disorder, major depressive disorder, and even IQ. CDS and the IN dimension of ADHD both contribute to variance in social problems and, apparently, peer neglect, yet their contributions are independent or additive, not redundant. Similarly, the study by Becker, Luebbe, et al. (2013) found that the positive association of CDS with general social problems was apparently not due to disruptive social problems, given the association noted above with significantly lower rates of discipline in inpatient children. This relationship of CDS to social withdrawal persists even after controlling for demographic factors and comorbidity. Thus CDS contributes unique variance to certain areas of social impairment independent of other disorders, including ADHD.
Academic Functioning. Bauermeister, et al. (2012) found that both CDS and ADHD IN were each significantly and independently associated with lower academic achievement scores on testing after controlling for the other set of symptoms, whereas hyperactive-impulsive symptoms showed no such relationship. Markovich-Pilon et al. (2017) also found a weak negative relationship of CDS symptoms with academic achievement tests (r = -.22), albeit barely significant, but only for teacher-rated CDS symptoms. In contrast, three studies did not find an association of CDS symptoms with academic achievement tests after controlling for IQ and ADHD symptoms, or found it to be rather weak. Again, the source of the ratings of CDS may account for this disparity in results across studies on achievement tests.
A few studies found that CDS symptoms may be uniquely associated with deficient math performance. Again, the source of the ratings may be relevant to such findings in that they may be more evident in teacher- than parent-rated CDS symptoms.
Others have found some measures of CDS symptoms to be uniquely linked to problems with writing, but not with reading. However, once again, these relationships varied depending on source (parent vs. teacher ratings), with stronger associations evident in teacher- than in parent-rated CDS symptoms, just as Markovich-Pilon, et al. (2017) found (above).
In contrast to these mixed results using academic tests, far stronger associations have been found for CDS symptoms when ratings of academic impairment are used. Like Burns, et al. (2013), Camprodon-Rosanas, et al. (2016) noted this same relationship in Spanish children. Likewise, Flannery, Luebbe and Becker (2016) found CDS symptoms to be uniquely linked to poorer study skills and globally rated educational impairment. Such relationships are evident even after controlling for ADHD IN symptoms in the above and other studies. The results of the study by Tamm, Garner, et al. (2016) indicate that it is the Slow or Sluggish dimension of CDS symptoms and not the Daydreamy/Spacey dimension that is related to academic impairment ratings. Smith and Langberg (2017) found the same result in a study of teens with ADHD, particularly for parent ratings of CDS Slow dimension. In contrast, youth self-report in that study was more strongly predictive of internalizing symptoms. Further evidence of academic functioning difficulties being linked to high CDS symptoms comes from a recent study by Shelton and colleagues (Shelton, Addison, & Hartung, 2017). They found CDS symptoms uniquely associated with certain self-regulated learning (SRL) strategies in college students apart from the SRL deficits linked to ADHD symptoms. Likewise, Becker and colleagues found a relationship of CDS symptoms to poor academic functioning in college students (Becker, Langberg, Luebbe, Dvorsky, & Flannery, in press).
The reason for the differences in results concerning academic functioning is obvious. It is the type of measurement of academic functioning and achievement being used in the study. Ratings of academic functioning in daily life are linked in specific and unique ways to CDS symptoms independent of those related to ADHD that may not be evident when psychometric tests of certain academic achievement skills are studied for their relationship to CDS.
Why else might there be a disparity across these studies besides measurement type? It may arise from the fact that some studies selected their samples for ADHD first, and then within such samples examined those high and low in CDS symptoms or examined the relationship of CDS to other correlates. This can contaminate any findings for CDS with those results related to ADHD. Even so, when symptoms of ADHD are statistically removed, CDS appears to add unique variance to the prediction of academic problems and may make unique contributions to written language, organization problems, and homework specifically beyond the contribution of ADHD IN symptoms.
Sleep. Given that problems with alertness, remaining awake, and even daytime sleepiness have been used as symptoms to index the presence of CDS, it is not surprising that the condition has been challenged for being a mere proxy of hypersomnia, or excess sleepiness. So for CDS to be established as a unique condition from other psychiatric or psychological problems, its relative independence from daytime drowsiness and nighttime sleep problems needs to be established. Several recent studies have now done so. A recent study of 7,346 clinic-referred children evaluated this relationship. Parent and teacher ratings were used to assess ADHD, CDS, anxiety, and depression symptoms along with parent reports of sleep problems. Controlling for ADHD, anxiety, depression, age, and medication status, the study showed only difficulty waking was significantly related to CDS, but only that which was parent-rated and not teacher-rated. This relationship was primarily with the sleepy/sluggish dimension of CDS, as one might expect. Another recent study examined 325 children referred to a sleep disorders clinic at a major children’s hospital. It found that CDS was weakly linked to most measures of sleep, but moderately correlated (.33-.53) with daytime sleepiness after controlling for child demographic factors, corroborating the Koriakin, et al. (2015) findings. Further corroboration comes from a recent study of children referred to a Canadian ADHD clinic in which, again, only parent-rated CDS, but not teacher-rated, was associated with daytime sleepiness, but not nighttime sleeping problems or duration. Thus, only parent-rated CDS symptoms in children, especially its Sleepy/Sluggish dimension, may share about 10%-30% of its variance with daytime sleepiness. This is not true of teacher-rated CDS. The finding for parent ratings is not surprising, given the inclusion of some items pertaining to sleepy/drowsy in CDS ratings.
A study of college students likewise finds a significant association of CDS symptoms, but not ADHD Inattention, with poorer sleep quality and increased nighttime sleep disturbance. Yet both CDS and ADHD Inattention were associated with greater daytime dysfunction above and beyond those nighttime sleep disturbances. In contrast, ADHD Hyperactivity symptoms were linked to poorer sleep quality, longer sleep latency, shorter sleep duration, and more use of sleep medications. But the small degree of the association in these studies, even if significant, means that CDS is not simply serving as a proxy for hypersomnia, daytime sleepiness, or sleeping difficulties, although it may be comorbid with and possibly contributory to both nighttime sleep problems and daytime dysfunction.
Driving. Only one study to date has examined the relationship of CDS to driving problems, and this was in 16- to 18-year-old adolescents with a history of chronically short sleep (5-7 hours). Results found that parent reported (but not self-reported) symptoms of CDS were significantly associated with driving violations (self-reported). In contrast, ADHD symptoms show a more marked and pervasive adverse relationship to various measures of driving performance and outcomes. Of interest to clinicians is that a treatment program intended to increase sleep time in these adolescents resulted in both a reduction in CDS symptoms and in fewer driving problems.
Global Impairment. In addition to social and academic domains, my own national surveys of children and adults included a measure of 15 domains of impairment. Cases were sorted into those who had CDS only, those with ADHD only, those with both conditions, and the remainder serving as the community control group. CDS cases were more impaired in all domains than control cases, having their greatest difficulties in Community-Leisure domains more than in Home-School (work) domains. In contrast, while ADHD cases were also impaired across all domains, their greatest difficulties occurred in Home-School domains. Moreover, ADHD was associated with more pervasive impairment. That is, both ADHD groups (alone and combined with CDS) experienced impairment in at least twice as many of the 15 domains as did CDS cases. The results also showed that ADHD symptom dimensions, especially inattention, contributed markedly more variance to impairment in the Home-School domains than did hyperactive-impulsive or CDS dimensions. By contrast, the hyperactive-impulsive dimension contributed more variance to Community-Leisure impairments, while CDS also did so but to a far lesser extent. When looking at individual domains for children among the 15 rated, CDS was not found to be more impairing than ADHD in educational settings, at least as rated by parents, consistent with other research discussed above. When ADHD and CDS symptoms were regressed onto the Community-Leisure and Home-School impairment summary scores, results found that both contributed uniquely to impairment, although ADHD accounted for a greater proportion of variance in each summary score. Hence, in children, CDS is a less impairing disorder than ADHD, but hardly benign.
The adult survey by Barkley (2012a) also used a rating scale of impairment in 15 domains more appropriate to adults. Both the CDS-only and ADHD-only groups were more impaired than the control group, but did not differ in this respect in overall mean impairment. A somewhat different pattern was evident for the percentage of domains in which impairment occurred (pervasiveness). Here, both of the ADHD groups (ADHD alone, ADHD+CDS) were impaired in more domains than was the CDS-only group and the control group. The results further revealed that the CDS-only group was also impaired in more domains than the control adults, but not to the degree evident in the ADHD groups. These results are consistent with the more recent large-scale study of college students by Becker and colleagues (2017) in that impairment is certainly linked to CDS in more distinct ways than is ADHD, but may not be so impairing as ADHD. A separate study of college students also found CDS to be uniquely linked to global impairment as well as in the specific domains of education, work, money and finances, managing household tasks and chores, community activities, and social situations, despite controlling for ADHD symptoms and anxiety and depression ratings.
It is noteworthy that in both of my large U.S. studies, when comorbid, CDS + ADHD disorders were additive in their impact on impairment ratings. That is, the combination of disorders resulted in far more severe impairment and more domains of impairment than either disorder alone.
Quality of Life. Combs and colleagues have also studied the linkage of CDS to some aspects of impairment in large adult community samples (Combs, Canu, Broman, & Nieman, 2013; Combs, Canu, Broman-Fulks, Rocheleau, & Nieman, 2012). In one study, the authors evaluated the contribution of both ADHD and CDS symptoms to a quality of life (QOL) measure. Findings indicated that each set of symptoms contributed unique variance to negative QOL ratings after controlling for the other set of symptoms as well as for anxiety, depression, and some demographic factors. The second study found much the same results for the association of ADHD and CDS with self-reported stress in adults. All of the above suggests that CDS is associated with distinct impairments in various domains of functioning from those associated with ADHD and contributes unique effects to impairment beyond that accounted for by ADHD.
Etiology
Very few studies have been done on the etiologies of CDS. A study by Moruzzi, Rijsdijk, and Battaglia (2014) examined the heritability of CDS using a very small set of items. CDS was substantially though moderately heritable with genetic factors accounting for the majority of variance in symptoms. However, a greater proportion of its variance was attributable to nonshared environmental factors than was the case for ADHD symptoms, which were even more substantially accounted for by genetic factors. And CDS shared about half of its genetic contribution with that of ADHD, implying some shared underlying genetic contributions but some unique to each disorder as well. Given the limited CDS item set, findings might have been different (larger heritability factor) had a longer, and hence more reliable, CDS scale been employed (because unreliability of measurement is clustered with the nonshared environment estimate).
Several studies observed a link between CDS symptoms and exposure to environmental toxins. One study observed that CDS was associated with prenatal alcohol exposure. Another noted the association of this symptom dimension with maternal smoking during pregnancy and also second-hand smoke exposure after birth. It has also been seen as a treatment emergent side effect along with lower IQ and lower academic achievement in acute lymphoblastic leukemia.
One neuro-imaging study has been reported in which CDS symptoms were more strongly associated with abnormal activity in posterior networks related to impaired orienting and shifting of attention as opposed to abnormalities in frontal-parietal (executive) networks so often evident in ADHD. The findings support a degree of differentiation between CDS symptoms and ADHD symptoms in the performance of controlled cognitive activities. Yet one study doesn’t make a definitive conclusion here.
And so it seems that like ADHD, CDS may turn out to have multiple etiologies. Most causes may fall in the realm of neurobiological and genetic factors, perhaps less strongly than with ADHD. We sorely need more research that uses neuro-imaging as well as more behavioral genetic and molecular genetic studies on the nature of CDS in comparison to other disorders, especially ADHD. However, researchers must take care to control for the overlap of CDS with ADHD. Not doing so will contaminate any findings with ADHD related results.
What is the Underlying Mental Dysfunction in CDS?
We simply do not know. It is possible that CDS represents a dysfunction in the focus/execute component of attention in Mirsky’s (1996) model of attention components. It is also possible that CDS is a form of hypersomnia or arousal disorder, given that some dimensions of CDS identified in past research include symptoms of sleepiness, low arousal or energy, or drowsiness. But this seems unlikely in view of recent evidence in college students that while both CDS and ADHD were significantly associated with daytime sleepiness, that sleepiness formed a distinct factor from those representing CDS and ADHD. So CDS is not just another label or proxy for hypersomnia, but it does have a significant association with daytime sleepiness even after controlling for ADHD, anxiety, and depression symptoms.
Could CDS be a form of pathological mind-wandering or maladaptive daydreaming? The fact that the only neuro-imaging study linked CDS symptoms to posterior regions that are implicated in the Default Mode Network and that this network has a close association with mind-wandering suggests this possibility. Research shows that mind-wandering is commonplace and advantageous under certain conditions. It arises when a primary task being performed demands little EF capacity and thus allows the contemplative or problem-solving capacity of the EF system to focus on more salient personal concerns. The latter then becomes a secondary task that is engaged while the individual performs the relatively automatic actions toward familiar goals (primary task) in the environment. When poorly regulated, however, mind-wandering can lead to adverse effects on performing EF tasks (perhaps due to reduced meta-awareness or self-monitoring of goal pursuit, diminished working memory capacity available for pursing the external goals, etc.). Excessive mind-wandering also can adversely affect academic performance. It would seem to be worthwhile for future research to investigate this possible association of CDS with pathological mind-wandering.
Other possibilities exist concerning the underlying nature of CDS. For instance, CDS could arise from a ruminative/obsessional disorder, perhaps being a milder variant of obsessive-compulsive disorder. Excessive and recurrent focusing on maladaptive thoughts might well lead to an attention problem resembling CDS. The same could be said for the attention problems evident in post-traumatic stress disorder. Or CDS could represent a deficit in motivation in which the person lacks not only energy but also initiative or self-motivation. I think that is unlikely given that research has not linked CDS to deficits in self-motivation as reflected on EF rating scales in children or adults once the overlap with ADHD symptoms is statistically removed. To date, it appears that CDS is associated with cognitive disengagement from monitoring and accurately processing external events with a greater focus of attention on mental events. These periods of spaceyness or disengagement from the environment may be associated with reduced physical activity and a sluggish response to environmental prompts to engage attention back to external events. For this reason, the recent work group report on CDS recommended the change in terminology (Becker et al., 2022).
Diagnosing CDS
During the initial evaluation of a child or adult, the suspicion of CDS can arise when there are complaints of inattention in the context of low or no symptoms of hyperactivity or impulsivity and where symptoms of passivity, hypo-activity, and even social withdrawal are evident. Clinicians can then use rating scales that directly assess CDS symptoms to evaluate the extent to which such symptoms are statistically abnormal relative to population norms, thus revealing the extent of developmental deviance.
There is no official set of diagnostic criteria for CDS. But my own research suggests that if parents endorse at least 3 or more of the 12 symptoms of CDS discussed earlier on the BSCTS-CA rating scale, and they occur often or frequently, this represents the 93rd percentile for the population. That is a traditional index of clinical significance and statistical deviance. That combined with evidence of impairment from the symptoms could be used for the time being as unofficial diagnostic criteria for CDS in children. It would identify about 5% of the U.S. child population as having this condition.
In the case of an adult, the symptom threshold would be 5 out of the 9 symptoms used in my study of adults and reflected on an adult CDS rating scale. When coupled with evidence of impairment in one or more major life activities, such as may be shown on normed rating scales of impairment, one can make an unofficial diagnosis of CDS.
Treatment of CDS
As with the etiology of CDS, there exist only a few studies on possible treatments for CDS, all in children. Early studies on stimulants (methylphenidate, or MPH) for treating ADHD I-type cases did not find them to be particularly effective in improving the inattention linked to CDS, although they were not harmful either. My own study found a modest positive response to MPH, mainly at low doses, but with only 20% of cases remaining on this medication after a double-blind, placebo-controlled trial. That contrasted sharply with the fact that the vast majority of ADHD-C children stayed on that medication and experienced a greater degree of improvement. But no stimulant medication studies have been done specifically in CDS cases. Even so, a recent research presentation (January, 2017) reported that higher CDS symptoms predicted a poorer response to MPH, consistent with these earlier studies on ADD-H or the Predominantly Inattentive Type of ADHD.
Only one study to date has examined a non-stimulant ADHD medication for treating CDS symptoms specifically. It found that the norepinephrine reuptake inhibitor atomoxetine was effective at reducing CDS symptoms in patients having both ADHD and dyslexia, ADHD only, and dyslexia only. The reduction in CDS symptoms remained evident even after statistically controlling for the overlap of with those of ADHD symptoms and also improved CDS symptoms in the group with dyslexia only.
What other medications might work to treat CDS? Given its overlap with anxiety and depression, perhaps SSRIs could be a possible treatment. Would an activating antidepressant (such as fluoxetine, sertraline, venlafaxine, or bupropion) reduce the observed sluggishness and boost alertness? Some clinicians have used Luvox for management of pathological mind-wandering, given its effects on obsessional thinking, but it is not clear that such thinking is the case in CDS. Given that CDS is associated with hypersomnia or daytime sleepiness, should one consider investigating the use of anti-narcoleptics, such as modafinil? It seems to me that the alpha-2 agonist guanfacine XR used for management of ADHD might be worth investigating for CDS, yet its side effects of sleepiness could be counter-productive in view of the sluggish/sleepy features seen in CDS. There is great opportunity for research here to explore possible medications for the management of this condition.
Just two studies of behavior modification methods have been done to date and these were done with Predominantly Inattentive ADHD cases, many of whom had CDS. It showed a good response of children with CDS symptoms to traditional home and school behavior management methods when targeted to the specific symptoms of children with CDS. Although it did not use CDS cases specifically, one study of social skills training found that children with ADHD IN type (who are more likely to have CDS) improved more in their assertion skills than did ADHD-C type cases. Yet neither ADHD type improved in other domains of social skills. Cognitive behavioral therapy has not been shown to be useful for ADHD. But it has proven useful for cases of anxiety and/or depression. I believe it may be worth exploring as a possible intervention for CDS, given the higher than expected comorbidity between these disorders. In view of the distinct symptoms and impairments of CDS relative to ADHD, treatments for ADHD cannot be automatically assumed to work for CDS, nor can those treatments that have failed for ADHD be thus ruled out for CDS.
Conclusions
- Cognitive Disengagement Syndrome (CDS) is an impairment of attention in daydreamy, hypoactive-appearing individuals, which first presents in childhood. It is characterized by a cognitive dimension of symptoms comprising daydreaming, sleepy, staring, “spaciness,” and mental fogginess and confusion, along with a motor dimension of slow movement, hypoactivity, lethargy, and passivity.
- The symptom dimensions forming CDS are distinct from, yet partially correlated, with those forming ADHD, especially its IN dimension.
- To avoid giving offense to patients having the condition and their families, as well as to not imply that the cognitive deficit in CDS is known, the condition should be called by a more benign label, such as Concentration Deficit Disorder or CDD.
- The history of CDS in the medical literature probably dates back to Alexander Crichton in 1798 or at the very least, to 1984 as found in research exploring the validity of ADD without Hyperactivity created in DSM-III.
- At this time, it exists only as a research entity that has yet to debut in diagnostic literature.
- SCT is associated with significant impairment, most reliably in social impairment, primarily social withdrawal. It also makes some contribution to difficulties with academic performance in children, and by adulthood even more so. It also is associated in adults with impairment in occupational functioning.
- SCT is also significantly associated with risk for internalizing symptoms and especially depression, and likely anxiety as well.
- It has no or even a negative relationship to ODD (and hence likely has no or even a negative relationship to CD, substance use disorders or antisocial personality).
- The etiologies of CDS are not well studied, but some evidence suggests a strong heritability to the disorder, but not as much so as seen in ADHD. CDS may also be associated with fetal alcohol exposure and with the treatment of acute lymphoblastic leukemia. Perhaps abnormal activity in the brain’s Default Mode Network may be linked to CDS, as one study suggests.
- Evidence supports the view that CDS is distinct from ADHD and not a subtype of it. But the two conditions can overlap in nearly half of all cases of each.
- Future diagnostic taxonomies, such as the DSM, should create a higher-order category of Attention Disorders (ADs) under which one would then break out ADHD and CDS as separate, semi-distinct conditions much like is done now for the supra-category of Learning Disabilities (LDs), rather than continue the mistaken view that CDS is a subtype of ADHD.
- Very little research has been done on treatments for CDS, and so no conclusions concerning its management can be made at this time.
REFERENCES on ADHD
(Those references specifically related to health outcomes and to CDS are in lists which follow this general one.)
Accardo, P. J., Blondis, T. A., Whitman, B. Y., & Stein, M. A. (2000).
Attention Deficits and Hyperactivity in Children and Adults. New York:
Marcel Dekker.
Achenbach, T. M. (1991). Manual for the Revised Child Behavior Profile and
Child Behavior Checklist. Burlington, VT: Author.
Achenbach, T. M., & Edelbrock, C. S. (1983). Manual for the Child Behavior
Profile and Child Behavior Checklist. Burlington, VT: Achenbach (author).
Achenbach, T. M., & Edelbrock, C. S. (1987). Empirically based assessment
of the behavioral/emotional problems of 2 and 3 year-old children.
Journal of Abnormal Child Psychology, 15, 629-650.
Achenbach, T. M., McConaughy, S. H., & Howell, C. T. (1987). Child/adolescent
behavioral and emotional problems: Implications of cross-informant correlations
for situational specificity. Psychological Bulletin, 101, 213-232.
Altepeter, T. S., & Breen, M. J. (1992). Situational variation in problem
behavior at home and school in attention deficit disorder with hyperactivity:
A factor analytic study. Journal of Child Psychology and Psychiatry, 33, 741-748.
Altink, M. E., Slaats-Willemse, D., Rommelse, N. N. J., Buschgens C., Fliers, E. A., Arias-Vasquez, A., Xu, X., Franke, B., Sergeant, J. A., Faraone, S. C., & Buitelaar, J. K. (2009). Effects of maternal and paternal smoking on attentional control in children with and without ADHD. European Child and Adolescent Psychiatry, 18, 465-475.
American Psychiatric Association. (1968). Diagnostic and statistical manual
of mental disorders (2nd ed.). Washington, DC: Author.
American Psychiatric Association. (1980). Diagnostic and statistical manual
of mental disorders (3rd ed.). Washington, DC: Author.
American Psychiatric Association. (1987). Diagnostic and statistical manual
of mental disorders (3rd ed., rev.). Washington, DC: Author.
American Psychiatric Association. (1994/2000). Diagnostic and statistical manual
of mental disorders (4th ed.). Washington, DC: Author.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
Anderson, C. A., Hinshaw, S. P., & Simmel, C. (1994). Mother-child
interactions in ADHD and comparison boys: Relationships with overt and covert
externalizing behavior. Journal of Abnormal Child Psychology, 22, 247-265.
Angold, A., Costello, E. J., & Erkanli, A. (1999). Comorbidity.
Journal of Child Psychology and Psychiatry, 40, 57-88.
Antrop, I., Roeyers, H., Van Oost, P., & Buysse, A. (2000). Stimulant
seeking and hyperactivity in children with ADHD. Journal of Child Psychology
and Psychiatry, 41, 225-231.
Applegate, B., Lahey, B. B., Hart, E. L., Waldman, I., Biederman, J., Hynd,
G. W., Barkley, R. A., Ollendick, T., Frick, P. J., Greenhill, L., McBurnett,
K., Newcorn, J., Kerdyk, L., Garfinkel, B., & Shaffer, D. (1997). Validity
of the age-of-onset criterion for ADHD: A report of the DSM-IV field trials.
Journal of American Academy of Child and Adolescent Psychiatry, 36, 1211-1221.
Aronen, E. T., Paavonen, J., Fjallberg, M., Soininen, M., Torronen, J. (2000).
Sleep and psychiatric symptoms in school-age children. Journal of the
American Academy of Child and Adolescent Psychiatry, 39, 502-508.
August, G. J., & Stewart, M. A. (1983). Family subtypes of childhood hyperactivity.
Journal of Nervous and Mental Disease, 171, 362-368.
August, G. J., Stewart, M. A., & Holmes, C. S. (1983). A four-year
follow-up of hyperactive boys with and without conduct disorder. British
Journal of Psychiatry, 143, 192-198.
Aylward, E. H., Reiss, A. L., Reader, M. J., Singer, H. S., Brown, J. E., &
Denckla, M. B. (1996). Basal ganglia volumes in children with attention-deficit
hyperactivity disorder. Journal of Child Neurology, 11, 112-115.
Babinski, D. E. (2024). Sex differences in ADHD: Review and priorities for future research. Current Psychiatry Reports, 26, 151-156.
Baddeley, A. (2001). Is working memory still working? American Psychologist.
Ball, J. D., & Koloian, B. (1995). Sleep patterns among ADHD children.
Clinical Psychology Review, 15, 681-691.
Ball, J. D., Tiernan, M., Janusz, J., & Furr, A. (1997). Sleep patterns
among children with attention-deficit hyperactivity disorder: A reexamination
of parent perceptions. Journal of Pediatric Psychology, 22, 389-398.
Baloh, R., Sturm, R., Green, B., & Gleser, G. (1975). Neuropsychological
effects of chronic asymptomatic increased lead absorption. Archives of Neurology,
32, 326-330.
Banaschewski, T., Becker, K., Scherag, S., Franke, B., & Coghill, D. (2010). Molecular genetics of attention-deficit/hyperactivity disorder: an overview. European Child and Adolescent Psychiatry, 19, 237-257.
Barkley, R. A. (1985). The social interactions of hyperactive children: Developmental
changes, drug effects, and situational variation. In R. McMahon & R. Peters
(Eds.), Childhood disorders: Behavioral-developmental approaches (pp.
218-243). New York: Brunner/Mazel.
Barkley, R. A. (1988). The effects of methylphenidate on the interactions of
preschool ADHD children with their mothers. Journal of the American Academy
of Child and Adolescent Psychiatry, 27, 336-341.
Barkley, R. A. (1989a). The problem of stimulus control and rule-governed behavior
in children with attention deficit disorder with hyperactivity. In J. Swanson
& L. Bloomingdale (Eds.), Attention deficit disorders (pp. 203-234).
New York: Pergamon.
Barkley, R. A. (1989b). Hyperactive girls and boys: Stimulant drug effects
on mother-child interactions. Journal of Child Psychology and Psychiatry,
30, 379-390.
Barkley, R. A. (1990). Attention-deficit hyperactivity disorder: A handbook
for diagnosis and treatment. New York: Guilford Press.
Barkley, R. A. (1994). Impaired delayed responding: A unified theory of attention
deficit hyperactivity disorder. In D. K. Routh (Ed.), Disruptive behavior disorders:
Essays in honor of Herbert Quay (pp. 11-57). New York: Plenum Press.
Barkley, R. A. (1997a). Behavioral inhibition sustained, attention, and executive
functions: Constructing a unifying theory of ADHD. Psychological Bulletin, 121,
65-94.
Barkley, R. A. (1997b). ADHD and the nature of self-control. New
York: Guilford
Barkley, R. A. (1999a). Response inhibition in attention deficit hyperactivity
disorder. Mental Retardation and Developmental Disabilities Research Reviews,
5, 177-184.
Barkley, R. A. (1999b). Theories of attention-deficit/hyperactivity disorder.
In H. Quay & A. Hogan (Eds.), Handbook of disruptive behavior disorders
(pp. 295-316). New York: Plenum.
Barkley, R. A. (2001a). The inattentive type of ADHD as a distinct disorder:
What remains to be done. Clinical Psychology: Science and Practice, 8,
489-493.
Barkley, R. A. (2001b). Genetics of childhood disorders: XVII. ADHD,
Part I: The executive functions and ADHD. Journal of the American Academy
of Child and Adolescent Psychiatry, 39, 1064-1068.
Barkley, R. A. (2001c). The executive functions and self-regulation:
An evolutionary neuropsychological perspective. Neuropsychology Review,
11, 1-29.
Barkley, R. A. (2006). Attention-deficit hyperactivity disorder: A handbook
for diagnosis and treatment (3rd ed.). New York: Guilford Press.
Barkley, R. A. (2010). Deficient emotional self-regulation is a core component of ADHD. Journal of ADHD and Related Disorders, 1, 5-37.
Barkley, R. A. (2011a). The Barkley Adult ADHD Rating Scale – IV. New York: Guilford. Press.
Barkley, R. A. (2011b). The Barkley Functional Impairment Scale. New York: Guilford. Press.
Barkley, R. A. (2011c). The Barkley Deficits in Executive Functioning Scale. New York: Guilford Press.
Barkley, R. A. (2012a). Distinguishing sluggish cognitive tempo from attention deficit hyperactivity disorder in adults. Journal of Abnormal Psychology. Advance online publication. doi: 10.1037/a0023961
Barkley, R. A. (2012b). Distinguishing sluggish cognitive tempo from ADHD in children and adolescents: executive functioning, impairment, and comorbidity. Journal of Clinical Child and Adolescent Psychology. DOI:10.1080/15374416.2012.734259
Barkley, R. A. (2012c). The Barkley Deficits in Executive Functioning Scale – Children and Adolescents. New York: Guilford Press.
Barkley, R. A. (2012d).The executive functions: What they are, how they work, and why they evolved. . New York: Guilford Press.
Barkley, R. A. (2013). Defiant Children: A Clinician’s Manual for Parent Training (3rd ed.). New York: Guilford Press.
Barkley, R. A. (2022). Treating ADHD in children and adolescents: What every clinician needs to know. New York: Guilford Press.
Barkley, R. A., Anastopoulos, A. D., Guevremont, D. G., & Fletcher, K.
F. (1991). Adolescents with attention deficit hyperactivity disorder: Patterns
of behavioral adjustment, academic functioning, and treatment utilization. Journal
of the American Academy of Child and Adolescent Psychiatry, 30, 752-761.
Barkley, R. A., Anastopoulos, A. D., Guevremont, D. G., & Fletcher, K.
F. (1992). Adolescents with attention deficit hyperactivity disorder: Mother-adolescent
interactions, family beliefs and conflicts, and maternal psychopathology. Journal
of Abnormal Child Psychology, 20, 263-288.
Barkley, R. A., & Biederman, J. (1997). Towards a broader definition
of the age of onset criterion for attention deficit hyperactivity disorder.
Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1204-1210.
Barkley, R. A. & Cox, D. J. (2007). A Review of Driving Risks and
Impairments Associated with Attention-Deficit/Hyperactivity Disorder and the
Effects of Stimulant Medication on Driving Performance. Journal of Safety
Research, 38, 113-128.
Barkley, R. A., & Cunningham, C. E. (1979a). Stimulant drugs and activity
level in hyperactive children. American Journal of Orthopsychiatry, 49, 491-499.
Barkley, R. A., & Cunningham, C. E. (1979b). The effects of methylphenidate
on the mother-child interactions of hyperactive children. Archives of
General Psychiatry, 36, 201-208.
Barkley, R., Cunningham, C., & Karlsson, J. (1983). The speech of hyperactive
children and their mothers: Comparisons with normal children and stimulant drug
effects. Journal of Learning Disabilities, 16, 105-110.
Barkley, R. A., DuPaul, G. J., & McMurray, M.B. (1990). A comprehensive
evaluation of attention deficit disorder with and without hyperactivity. Journal
of Consulting and Clinical Psychology, 58, 775-789.
Barkley, R. A., & Edelbrock, C. S. (1987). Assessing situational variation
in children’s behavior problems: The Home and School Situations Questionnaires.
In R. Prinz (Ed.), Advances in behavioral assessment of children and families
(Vol. 3, pp. 157-176). Greenwich, CT: JAI Press.
Barkley, R. A. Edwards, G., Laneri, M., Fletcher, K., & Metevia, L.
(2001). Executive functioning, temporal discounting, and sense of time
in adolescents with attention deficit hyperactivity disorder and oppositional
defiant disorder. Journal of Abnormal Child Psychology, 29, 541-556.
Barkley, R. A. & Fischer, M. (2010). The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. Journal of the American Academy of Child and Adolescent Psychiatry, 49, 503-513.
Barkley, R. A., Fischer, M., Edelbrock, C. S., & Smallish, L. (1990). The
adolescent outcome of hyperactive children diagnosed by research criteria: I.
An 8 year prospective follow-up study. Journal of the American Academy of Child
and Adolescent Psychiatry, 29, 546-557.
Barkley, R. A., Fischer, M., Edelbrock, C. S., & Smallish, L. (1991). The
adolescent outcome of hyperactive children diagnosed by research criteria: III.
Mother-child interactions, family conflicts, and maternal psychopathology.
Journal of Child Psychology and Psychiatry, 32, 233-256.
Barkley, R. A., Fischer, M., Fletcher, K., & Smallish, L. (2002).
Persistence of attention deficit hyperactivity disorder into adulthood as a
function of reporting source and definition of disorder. Journal of Abnormal
Psychology, 111, 279-289.
Barkley, R. A., Fischer, M., Smallish, L., & Fletcher, K. (2003). Does the treatment of ADHD with stimulant medication contribute to illicit
drug use and abuse in adulthood? Results from a 15-year prospective study.
Pediatrics, 111, 109-121.
Barkley, R. A., Grodzinsky, G., & DuPaul, G. (1992). Frontal lobe functions
in attention deficit disorder with and without hyperactivity: A review and research
report. Journal of Abnormal Child Psychology, 20, 163-188.
Barkley, R. A., Guevremont, D. G., Anastopoulos, A. D., DuPaul, G. J., &
Shelton, T. L. (1993). Driving-related risks and outcomes of attention deficit
hyperactivity disorder in adolescents and young adults: A 3-5 year follow-up
survey. Pediatrics, 92, 212-218.
Barkley, R. A., Karlsson, J., & Pollard, S. (1985). Effects of age on the
mother-child interactions of hyperactive children. Journal of Abnormal
Child Psychology, 13, 631-638.
Barkley, R. A., Karlsson, J., Pollard, S., & Murphy, J. V. (1985). Developmental
changes in the mother-child interactions of hyperactive boys: Effects
of two dose levels of Ritalin. Journal of Child Psychology and Psychiatry and
Allied Disciplines, 26, 705-715.
Barkley, R. A., Knouse, L. E., & Murphy, K. R. (2011). Correspondence and disparity in the self and other ratings of current and childhood symptoms and impairments in adults with ADHD. Psychological Assessment, 23, 437-446.
Barkley, R. A. & Murphy, K. R. (2010a). Deficient emotional self-regulation in adults with ADHD: The relative contributions of emotional impulsiveness and ADHD symptoms to adaptive impairments in major life activities. Journal of ADHD and Related Disorders, 1(4), 5-28.
Barkley, R. A., & Murphy, K. R. (2010b). Impairment in occupational functioning and adult ADHD: The predictive utility of executive function (EF) ratings vs. EF tests. Archives of Clinical Neuropsychology, 25, 157-173.
Barkley, R. A., Murphy, K. R., & Bush, T. (2001). Time perception
and reproduction in young adults with attention deficit hyperactivity disorder
(ADHD). Neuropsychology, 15, 351-360.
Barkley, R. A., Murphy, K. R., DuPaul, G. R., & Bush, T. (2002).
Driving in young adults with attention deficit hyperactivity disorder:
Knowledge, performance, adverse outcomes and the role of executive functions.
Journal of the International Neuropsychological Society, 8, 655-672.
Barkley, R. A., Murphy, K. R., & Fischer, M. (2008). ADHD in Adults:
What the Science Says. New York: Guilford Publications.
Barkley, R. A., Murphy, K. R., & Kwasnik, D. (1996a). Psychological
functioning and adaptive impairments in young adults with ADHD. Journal
of Attention Disorders, 1, 41-54.
Barkley, R. A., Murphy, K. R., & Kwasnik, D. (1996b). Motor vehicle
driving competencies and risks in teens and young adults with attention deficit
hyperactivity disorder. Pediatrics, 98, 1089-1095.
Barkley, R. A., & Peters, H. (2012). The earliest reference to ADHD in the medical literature? Melchior Adam Weikard’s description in 1775 of “Attention Deficit” (Mangel der Aufmerksamkeit, attentio volubilis). Journal of Attention Disorders, 16, 623-630.
Barkley, R. A., Shelton, T. L., Crosswait, C., Moorehouse, M., Fletcher, K., Barrett, S., Jenkins, L., & Metevia, L. (2002). Preschool children with high levels of disruptive behavior: Three-year outcomes as a function of adaptive disability. Development and Psychopathology, 14, 45-68.
Bate, A. J., Mathias, J. L., & Crawford, J. R. (2001). Performance
of the Test of Everyday Attention and standard tests of attention following
severe traumatic brain injury. The Clinical Neuropsychologist, 15, 405-422.
Bauermeister, J. J., Barkley, R. A., Bauermeister, J. A., Martinez, J. V., & McBurnett, K. (2011). Validity of the sluggish cognitive tempo, inattention, and hyperactivity symptom dimensions: neuropsychological and psychosocial correlates. Journal of Abnormal Child Psychology, online first, December 2011.
Bayliss, D. M., & Roodenrys, S. (2000). Executive processing and
attention deficit hyperactivity disorder: An application of the supervisory
attentional system. Developmental Neuropsychology, 17, 161-180.
Beauchaine, T. P., Katkin, E. S., Strassberg, Z., & Snarr, J. (2001).
Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder
from attention-deficit/hyperactivity disorder through concurrent assessment
of multiple autonomic states. Journal of Abnormal Psychology, 110, 610-624.
Becker, S. P., Willcutt, E. G., Leopold, D. R., Fredrick, J. W., Smith, Z. R., Jacobson, L. A., Burns, G. L., Mayes, S. D., Waschbusch, D. A., Froehlich, T. E., McBurnett, K., Servera, M., & Barkley, R. A. (2022). Report of a work group on sluggish cognitive tempo: Key research directions and a consensus change in terminology to Cognitive Disengagement Hypoactivity Syndrome. Journal of the American Academy of Child and Adolescent Psychiatry, 62, 629-645.
Befera, M., & Barkley, R. A. (1984). Hyperactive and normal girls and boys:
Mother-child interactions, parent psychiatric status, and child psychopathology.
Journal of Child Psychology and Psychiatry, 26, 439-452.
Beiser, M., Dion, R., & Gotowiec, A. (2000). The structure of attention-deficit
and hyperactivity symptoms among native and non-native elementary school children.
Journal of Abnormal Child Psychology, 28, 425-537.
Beitchman, J. H., Wekerle, C., & Hood, J. (1987). Diagnostic continuity
from preschool to middle childhood. Journal of the American Academy of Child
and Adolescent Psychiatry, 26, 694-699.
Bellani, M., Moretti, A., Perlini, C., & Brambilla, P. (2011). Langugae disturbances in ADHD. Epidemiology and Psychiatric Sciences, online first, doi:10.1017/S2045796011000527.
Benton, A. (1991). Prefrontal injury and behavior in children. Developmental
Neuropsychology, 7, 275-282.
Berk, L. E., & Potts, M. K. (1991). Development and functional significance
of private speech among attention-deficit hyperactivity disorder and normal
boys. Journal of Abnormal Child Psychology, 19, 357-377.
Bhatia, M. S., Nigam, V. R., Bohra, N., & Malik, S. C. (1991). Attention
deficit disorder with hyperactivity among paediatric outpatients. Journal of
Child Psychology and Psychiatry, 32, 297-306.
Biederman, J., Faraone, S. V., Keenan, K., & Tsuang, M. T. (1991). Evidence
of a familial association between attention deficit disorder and major affective
disorders. Archives of General Psychiatry, 48, 633-642.
Biederman, J., Faraone, S. V., & Lapey, K. (1992). Comorbidity of diagnosis
in attention-deficit hyperactivity disorder. In G. Weiss (Ed.), Child and adolescent
psychiatric clinics of North America: Attention-deficit hyperactivity disorder
(pp. 335-360). Philadelphia: Saunders.
Biederman, J., Faraone, S. V., Mick, E., Spencer, T., Wilens, T., Kiely, K.,
Guite, J., Ablon, J. S., Reed, E., Warburton, R. (1995). High risk for
attention deficit hyperactivity disorder among children of parents with childhood
onset of the disorder: A pilot study. American Journal of Psychiatry,
152, 431-435.
Biederman, J., Faraone, S. V., Mick, E., Williamson, S., Wilens, T. E., Spencer,
T. J., Weber, W., Jetton, J., Kraus, I., Pert, J., & Zallen, B. (1999).
Clinical correlates of ADHD in females: Findings from a large group of girls
ascertained from pediatric and psychiatric referral sources. Journal of
the American Academy of Child and Adolescent Psychiatry, 38, 966-975
Biederman, J., Faraone, S., Milberger, S., Curtis, S., Chen, L., Marrs, A.,
Ouellette, C., Moore, P., & Spencer, T. (1996). Predictors of persistence
and remission of ADHD into adolescence: Results from a four-year prospective
follow-up study. Journal of the American Academy of Child and Adolescent
Psychiatry, 35, 343-351.
Biederman, J., Keenan, K., & Faraone, S. V. (1990). Parent-based diagnosis
of attention deficit disorder predicts a diagnosis based on teacher report.
American Journal of Child and Adolescent Psychiatry, 29, 698-701.
Biederman, J., Milberger, S., Faraone, S. V., Guite, J., & Warburton, R.
(1994). Associations between childhood asthma and ADHD: Issues of psychiatric
comorbidity and familiality. Journal of the American Academy of Child and Adolescent
Psychiatry, 33, 842-848.
Biederman, J., Newcorn, J., & Sprich, S. (1991). Comorbidity of attention
deficit hyperactivity disorder with conduct, depressive, anxiety, and other
disorders. American Journal of Psychiatry, 148, 564-577.
Biederman, J., Wilens, T., Mick, E., Spencer, T., & Faraone, S. V.
(1999). Pharmacotherapy of attention-deficit/hyperactivity disorder reduces
risk for substance use disorder. Pediatrics, 104.
Biederman, J., Wozniak, J., Kiely, K., Ablon, S., Faraone, S., Mick, E., Mundy,
E., & Kraus, I. (1995). CBCL clinical scales discriminate prepubertal children
with structured-interview-derived diagnosis of mania from those with ADHD. Journal
of the American Academy of Child and Adolescent Psychiatry, 34, 464-471.
Bijur, P., Golding, J., Haslum, M., & Kurzon, M. (1988). Behavioral predictors
of injury in school-age children. American Journal of Diseases of Children,
142, 1307-1312.
Block, G. H. (1977) Hyperactivity: a cultural perspective. Journal of
Learning Disabilities, 110, 236-240.
Borger, N., & van der Meere, J. (2000). Visual behaviour of ADHD
children during an attention test: An almost forgotten variable. Journal
of Child Psychology and Psychiatry, 41, 525-532.
Braaten, E. B., & Rosen, L. A. (2000). Self-regulation of affect
in attention deficit - hyperactivity disorder (ADHD) and non-ADHD boys:
Differences in empathic responding. Journal of Consulting and Clinical
Psychology, 68, 313-321.
Breen, M. J. (1989). Cognitive and behavioral differences in ADHD boys and
girls. Journal of Child Psychology and Psychiatry, 30, 711-716.
Breggin, P. (1998). Talking back to Ritalin. Monroe, ME: Common Courage
Press.
Breslau, N., Brown, G. G., DelDotto, J. E., Kumar, S., Exhuthachan, S., Andreski,
P., & Hufnagle, K. G. (1996). Psychiatric sequelae of low birth weight
at 6 years of age. Journal of Abnormal Child Psychology, 24, 385-400.
Bronowski, J. (1977). Human and animal languages. In A sense of the future
(pp. 104-131). Cambridge, MA: MIT Press.
Brown, T. E. (2009). ADHD Comorbidities: Handbook for ADHD Complications in Children and Adults. Washington, DC: American Psychiatric Press.
Bu-Haroon, A., Eapen, V., & Bener, A. (1999). The prevalence of hyperactivity
symptoms in the United Arab Emirates. Nordic Journal of Psychiatry, 53,
439-442.
Buhrmester, D., Camparo, L., Christensen, A., Gonzalez, L. S., & Hinshaw,
S. P. (1992). Mothers and fathers interacting in dyads and triads with normal
and hyperactive sons. Developmental Psychology, 28, 500-509.
Burke, J. D., Loeber, R., & Lahey, B. B. (2001). Which aspects of ADHD
are associated with tobacco use in early adolescence? Journal of Child Psychology
and Psychiatry, 493-502.
Burks, H. (1960). The hyperkinetic child. Exceptional Children, 27, 18.
Burns, G. L., Boe, B., Walsh, J. A., Sommers-Flannagan, R., & Teegarden,
L. A. (2001). A confirmatory factor analysis on the DSM-IV ADHD and ODD
symptoms: What is the best model for the organization of these symptoms?
Journal of Abnormal Child Psychology, 29, 339-349.
Burns, G. L., & Walsh, J. A. (in press). The influence of ADHD-hyperactivity/impulsivity
symptoms on the development of oppositional defiant disorder symptoms in a two-year
longitudinal study. Journal of Abnormal Child Psychology.
Burt, S. A., Larsson, H., Lichtenstein, P., & Klump, K. I. (2012). Additional evidence against shared environmental contributions to attention-deficit/hyperactivity disorder. Behavior Genetics, online first, DOI 10.1007/s10519-012-9545-y.
Cadesky, E. B., Mota, V. L., & Schachar, R. J. (2000). Beyond words:
how do children with ADHD and/or conduct problems process nonverbal information
about affect? Journal of the American Academy of Child and Adolescent
Psychiatry,39, 1160-1167.
Cadoret, R. J., & Stewart, M. A. (1991). An adoption study of attention
deficit/hyperactivity/aggression and their relationship to adult antisocial
personality. Comprehensive Psychiatry, 32, 73-82.
Campbell, S. B. (1990). Behavior problems in preschool children. New York:
Guilford Press.
Campbell, S. B., March, C. L., Pierce, E. W., Ewing, L. J., & Szumowski,
E. K. (1991). Hard-to-manage preschool boys: Family context and the stability
of externalizing behavior. Journal of Abnormal Child Psychology, 19, 301-318.
Campbell, S. B., Schleifer, M., & Weiss, G. (1978). Continuities in maternal
reports and child behaviors over time in hyperactive and comparison groups.
Journal of Abnormal Child Psychology, 6, 33-45.
Campbell, S. B., Szumowski, E. K., Ewing, L. J., Gluck, D. S., & Breaux,
A. M. (1982). A multidimensional assessment of parent-identified behavior problem
toddlers. Journal of Abnormal Child Psychology, 10, 569-592.
Cantwell, D. (1975). The hyperactive child. New York: Spectrum.
Cantwell, D. P., & Baker, L. (1992). Association between attention deficit-hyperactivity
disorder and learning disorders. In S. E. Shaywitz & B. A. Shaywitz (Eds.),
Attention deficit disorder comes of age: Toward the twenty-first century (pp.
145-164). Austin, TX: Pro-ed.
Carlson, C. L., Lahey, B. B., & Neeper, R. (1986). Direct assessment
of the cognitive correlates of attention deficit disorders with and without
hyperactivity. Journal of Behavioral Assessment and Psychopathology, 8,
69-86.
Carlson, C. L., & Mann, M. (2002). Sluggish cognitive tempo
predicts a different pattern of impairment in the Attention Deficit Hyperactivity
Disorder, Predominantly Inattentive Type. Journal of Clinical Child
and Adolescent Psychology, 31, 123-129.
Carlson, C. L., & Tamm, L. (2000). Responsiveness of children with
attention deficit-hyperactivity disorder to reward and response cost: Differential
impact on performance and motivation. Journal of Consulting and Clinical
Psychology, 68, 73-83.
Carlson, C. L., Tamm, L., & Gaub, M. (1997). Gender differences in
children with ADHD, ODD, and co-occurring ADHD/ODD identified in a school population.
Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1706-1714.
Carlson, E. A., Jacobvitz, D., & Sroufe, L. A. (1995). A developmental
investigation of inattentiveness and hyperactivity. Child Development,
66, 37-54.
Carlson, G. A. (1990). Child and adolescent mania – diagnostic considerations.
Journal of Child Psychology and Psychiatry, 31, 331-342.
Carr, L., Henderson, J., & Nigg, J. T. (2010). Cognitive control and attentional selection in adolescents with ADHD versus ADD. Journal of Clinical Child and Adolescent Psychology, 39, 726-740.
Carte, E. T., Nigg, J. T., & Hinshaw, S. P. (1996). Neuropsychological
functioning, motor speed, and language processing in boys with and without ADHD.
Journal of Abnormal Child Psychology, 24, 481-498.
Casey, J. E., Rourke, B. P., & Del Dotto, J. E. (1996). Learning
disabilities in children with attention deficit disorder with and without hyperactivity.
Child Neuropsychology, 2, 83-98.
Casey, R. J. (1996). Emotional competence in children with externalizing
and internalizing disorders. In M. Lewis & M. W. Sullivan (Eds.),
Emotional development in atypical children (pp. 161-183). Mahwah, NJ:
Lawrence Erlbaum Associates.
Castellanos, F. X., Marvasti, F. F., Ducharme, J. L., Walter, J. M., Israel,
M. E., Krain, A., Pavlovsky, C., & Hommer, D. W. (2000). Executive
function oculomotor tasks in girls with ADHD. Journal of the American
Academy of Child and Adolescent Psychiatry, 39, 644-650.
Chadwick, O., Taylor, E., Taylor, A., Heptinstall, E., & Danckaerts, M.
(1999). Hyperactivity and reading disability: A longitudinal study of
the nature of the association. Journal of Child Psychology and Psychiatry,
40, 1039-1050
Chang, H. T., Klorman, R., Shaywitz, S. E., Fletcher, J. M., Marchione, K.
E., Holahan, J. M., Stuebing, K. K., Brumaghim, J. T., & Shaywitz, B. A.
(1999). Paired-associate learning in attention-deficit/hyperactivity disorder
as a function of hyperactivity-impulsivity and oppositional defiant disorder.
Journal of Abnormal Child Psychology, 27, 237-245.
Charach, A., Yeung, E., Climans, T., & Lillie, E. (2011). Childrenhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry, 50, 9-21.
Chess, S. (1960). Diagnosis and treatment of the hyperactive child. New York
State Journal of Medicine, 60, 2379- 2385.
Chilcoat, H. D., & Breslau, N. (1999).Pathways from ADHD to early drug
use. Journal of the American Academy of Child and Adolescent Psychiatry.
38, 1347-1354
Clark, C., Prior, M., & Kinsella, G. J. (2000). Do executive function
deficits differentiate between adolescents with ADHD and oppositional defiant/conduct
disorder? A neuropsychological study using the Six Elements Test and Hayling
Sentence Completion Test. Journal of Abnormal Child Psychology, 28, 405-414.
Clark, M. L., Cheyne, J. A., Cunningham, C. E., & Siegel, L. S. (1988).
Dyadic peer interaction and task orientation in attention-deficit-disordered
children. Journal of Abnormal Child Psychology, 16, 1-15.
Claude, D., & Firestone, P. (1995). The development of ADHD boys:
A 12-year follow-up. Canadian Journal of Behavioural Science, 27, 226-249.
Coghill, D., Nigg, J., Rothenberger, A., Sonuga-Barke, E., & Tannock, R. (2005). Wither causal models in the neuroscience of ADHD? Developmental Science, 8, 105-114.
Cohen, N. J., & Minde, K. (1983). The “hyperactive syndrome”
in kindergarten children: Comparison of children with pervasive and situational
symptoms. Journal of Child Psychology and Psychiatry, 24, 443-455.
Cohen, N. J., Sullivan, J., Minde, K., Novak, C., & Keens, S. (1983). Mother-child
interaction in hyperactive and normal kindergarten-aged children and the effect
of treatment. Child Psychiatry and Human Development, 13, 213-224.
Cohen, N. J., Vallance, D. D., Barwick, M., Im, N., Menna, R., Horodezky, N.
B., & Isaacson, L. (2000). The interface between ADHD and language
impairment: An examination of language, achievement, and cognitive processing.
Journal of Child Psychology and Psychiatry,41, 353-362.
Comings, D. E. (2000). Attention deficit hyperactivity disorder with
Tourette Syndrome. In T. E. Brown (Ed.), Attention-deficit disorders and
comorbidities in children, adolescents, and adults (pp. 363-392). Washington,
DC: American Psychiatric Press.
Connor, D. F. (2006). Stimulants. In R. A. Barkley (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (pp. 608-647). New York: Guilford.
Corkum, P. V., Beig, S., Tannock, R., & Moldofsky, H. (1997). Comorbidity:
The potential link between attention-deficit/hyperactivity disorder and sleep
problems. Paper presented at the annual meeting of the American Academy
of Child and Adolescent Psychiatry, Toronto, October.
Corkum, P., Moldofsky, H., Hogg-Johnson, S., Humphries, T., & Tannock,
R. (1999). Sleep problems in children with attention-deficit/hyperactivity
disorder: Impact of subtype, comorbidity, and stimulant medication. Journal
of the American Academy of Child and Adolescent Psychiatry, 38, 1285-1293.
Cortese, S., Faraone, S. V., Konofal, E., & Lecendreaux, M. (2009). Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 894-908.
Costello, E. J., Loeber, R., & Stouthamer-Loeber, M. (1991). Pervasive
and situational hyperactivity – Confounding effect of informant: A research
note. Journal of Child Psychology and Psychiatry, 32, 367-376.
Crichton, A. (1798). An inquiry into the nature and origin of mental derangement: Comprehending a concise system of the physiology and pathology of the human mind and a history of the passions and their effects. London: T. Cadell, Jr. and W. Davies. [Reprinted by AMS Press, New York, 1976.]
Cruickshank, B. M., Eliason, M., & Merrifield, B. (1988). Long-term
sequelae of water near-drowning. Journal of Pediatric Psychology, 13,
379-388.
Crystal, D. S., Ostrander, R., Chen, R. S., & August, G. J. (2001).
Multimethod assessment of psychopathology among DSM-IV subtypes of children
with attention-deficit/hyperactivity disorder: self, parent, and teacher reports.
Journal of abnormal Child Psychology, 29, 189-205.
Cuffe, S. P., McKeown, R. E., Jackson, K. L., Addy, C. L., Abramson, R., &
Garrison, C. Z. (2001). Prevalence of attention-deficit/hyperactivity
disorder in a community sample of older adolescents. Journal of the American
Academy of Child and Adolescent Psychiatry, 40, 1037-1044.
Cunningham, C. E., Benness, B. B., & Siegel, L. S. (1988). Family functioning,
time allocation, and parental depression in the families of normal and ADDH
children. Journal of Clinical Child Psychology, 17, 169-177.
Cunningham, C. E., & Siegel, L. S. (1987). Peer interactions of normal
and attention-deficit disordered boys during free-play, cooperative task, and
simulated classroom situations. Journal of Abnormal Child Psychology, 15, 247-
268.
Cunningham, C. E., Siegel, L. S., & Offord, D. R. (1985). A developmental
dose response analysis of the effects of methylphenidate on the peer interactions
of attention deficit disordered boys. Journal of Child Psychology and Psychiatry,
26, 955-971.
Dane, A. V., Schachar, R. J., & Tannock, R. (2000). Does actigraphy
differentiate ADHD subtypes in a clinical research setting? Journal of
the American Academy of Child and Adolescent Psychiatry, 39, 752-760.
Danforth, J. S., Barkley, R. A., & Stokes, T. F. (1991). Observations of
parent-child interactions with hyperactive children: Research and clinical
implications. Clinical Psychology Review, 11, 703-727.
Daugherty, T. K., & Quay, H. C. (1991). Response perseveration and
delayed responding in childhood behavior disorders. Journal of Child Psychology
and Psychiatry, 32, 453-461.
David, O. J. (1974). Association between lower level lead concentrations and
hyperactivity. Environmental Health Perspective, 7, 17-25.
Daviss, W. B. (2008). A review of co-morbid depression in pediatric ADHD: etiologies, phenomenology, and treatment. Journal of Children and Adolescent Psychopharmacology, 18, 565-571.
de la Burde, B., & Choate, M. (1972). Does asymptomatic lead exposure in
children have latent sequelae? Journal of Pediatrics, 81, 1088-1091.
de la Burde, B., & Choate, M. (1974). Early asymptomatic lead exposure
and development at school age. Journal of Pediatrics, 87, 638-642.
Demaray, M. K., & Elliot, S. N. (2001). Perceived social support
by children with characteristics of attention-deficit/hyperactivity disorder.
School Psychology Quarterly, 16, 68-90.
Demb, H. B. (1991). Use of Ritalin in the treatment of children with mental
retardation. In L. L. Greenhill & B. B. Osmon (Eds.), Ritalin: Theory and
patient management (pp. 155-170). New York: Mary Ann Liebert.
Denckla, M. B. (1994). Measurement of executive function. In G. R. Lyon (Ed.),
Frames of reference for the assessment of learning disabilities: New views on
measurement issues (pp. 117-142). Baltimore: Brookes.
Denckla, M. B., & Rudel, R. G. (1978). Anomalies of motor development in
hyperactive boys. Annals of Neurology, 3, 231-233.
Denckla, M. B., Rudel, R. G., Chapman, C., & Krieger, J. (1985).
Motor proficiency in dyslexic children with and without attentional disorders.
Archives of Neurology, 42, 228-231.
Diamond, A. (1990). The development and neural bases of memory functions as
indexed by the AB and delayed response task in human infants and infant monkeys.
In A. Diamond (Ed.), The development and neuronal bases of higher cognitive
functions. Annals of the New York Academy of Science, 608, 276-317.
Diamond, A. (2000). Close interrelation of motor development and cognitive
development and of the cerebellum and prefrontal cortex. Developmental
Psychology, 71, 44-56.
Diamond, A., Cruttenden, L., & Neiderman, D. (1994). AB with multiple wells:
1. Why are multiple wells sometimes easier than two wells? 2. Memory or memory
+ inhibition? Developmental Psychology, 30, 192-205.
Diaz, R. M., & Berk, L. E. (1992). Private speech: From social
interaction to self-regulation. Mahwah, NJ: Lawrence Erlbaum Associates,
Inc.
Dolphin, J. E., & Cruickshank, W. M. (1951). Pathology of concept formation
in children with cerebral palsy. American Journal of Mental Deficiency, 56,
386-392.
Douglas, V. I. (1972). Stop, look, and listen: The problem of sustained attention
and impulse control in hyperactive and normal children. Canadian Journal of
Behavioural Science, 4, 259-282.
Douglas, V. I. (1980). Higher mental processes in hyperactive children: Implications
for training. In R. Knights & D. Bakker (Eds.), Treatment of hyperactive
and learning disordered children (pp. 65-92). Baltimore: University Park
Press.
Douglas, V. I. (1983). Attention and cognitive problems. In M. Rutter (Ed.),
Developmental neuropsychiatry (pp. 280- 329). New York: Guilford Press.
Douglas, V. I., & Parry, P. A. (1983). Effects of reward on delayed reaction
time task performance of hyperactive children. Journal of Abnormal Child Psychology,
11, 313-326.
Douglas, V. I., & Parry, P. A. (1994). Effects of reward and non-reward
on attention and frustration in attention deficit disorder. Journal of Abnormal
Child Psychology, 22, 281-302.
Douglas, V. I., & Peters, K. G. (1978). Toward a clearer definition of
the attentional deficit of hyperactive children. In G. A. Hale & M. Lewis
(Eds.), Attention and the development of cognitive skills (pp. 173-248). New York: Plenum Press.
Doyle, A. E., Faraone, S. V., DuPre, E. P., & Biederman, J. (2001).
Separating attention deficit hyperactivity disorder and learning disabilities
in girls: A familial risk analysis. American Journal of Psychiatry, 158,
1666-1672.
Draeger, S., Prior, M., & Sanson, A. (1986). Visual and auditory attention
performance in hyperactive children: Competence or compliance. Journal of Abnormal
Child Psychology, 14, 411-424.
DuPaul, G. J. (1991). Parent and teacher ratings of ADHD symptoms: Psychometric
properties in a community-based sample. Journal of Clinical Child Psychology,
20, 245-253.
DuPaul, G. J., & Barkley, R. A. (1992). Situational variability of attention
problems: Psychometric properties of the Revised Home and School Situations
Questionnaires. Journal of Clinical Child Psychology, 21, 178-188.
DuPaul, G. J., Barkley, R. A., & Connor, D. F. (1998). Stimulants.
In RA Barkley, ed., Attention Deficit Hyperactivity Disorder: A Handbook for
Diagnosis and Treatment (pp. 510-551). New York: Guilford.
DuPaul, G. J., Gormley, M. J., & Laracy, S. D. (2012). Comorbidity of LD and ADHD: Implications of DSM-% for assessment and treatment. Journal of Learning Disabilities, online first, DOI: 10.1177/0022219412464351.
DuPaul, G. J., McGoey, K. E., Eckert, T. L., & VanBrakle, J. (2001).
Preschool children with attention-deficit/hyperactivity disorder: impairments
in behavioral, social, and school functioning. Journal of the American
Academy of Child and Adolescent Psychiatry,40, 508-515.
Ebaugh, F. G. (1923). Neuropsychiatric sequelae of acute epidemic encephalitis
in children. American Journal of Diseases of Children, 25, 89-97.
Edwards, F., Barkley, R., Laneri, M., Fletcher, K., & Metevia, L. (2001).
Parent-adolescent conflict in teenagers with ADHD and ODD. Journal of
Abnormal Child Psychology, 29, 557-572.
Erhardt, D., & Hinshaw, S. P. (1994). Initial sociometric impressions
of attention-deficit hyperactivity disorder and comparison boys: predictions
from social behaviors and from nonbehavioral variables. Journal of Consulting
and Clinical Psychology, 62, 833-842.
Ernst, M., Cohen, R. M., Liebenauer, L. L., Jons, P. H. & Zametkin, A.
J. (1997). Cerebral glucose metabolism in adolescent girls with attention-deficit/hyperactivity
disorder. Journal of the American Academy of Child and Adolescent Psychiatry,
36, 1399-1406.
Ernst, M., Liebenauer, L. L., King, A. C., Fitzgerald, G. A., Cohen, R. M.,
& Zametkin, A. J. (1994). Reduced brain metabolism in hyperactive girls.
Journal of the American Academy of Child and Adolescent Psychiatry, 33, 858-868.
Ernst, M., Zametkin, A. J., Matochik, J. A., Pascualvaca, D., Jons, P. H.,
& Cohen, R. M. (1999). High midbrain [18F]DOPA accumulation in children
with attention deficit hyperactivity disorder. American Journal of Psychiatry,
156, 1209-1215.
Fallone, G., Acebo, C., Arnedt, J. T., Seifer, R., Carskadon, M. A. (2001).
Effects of acute sleep restriction on behavior, sustained attention, and response
inhibition in children. Perceptual and Motor Skills, 93, 213-229.
Faraone, S. V., & Biederman, J. (1997). Do attention deficit hyperactivity
disorder and major depression share familial risk factors? Journal of
Nervous and Mental Disease, 185, 533-541.
Faraone, S. V., Biederman, J., Chen, W. J., Krifcher, B., Keenan, K., Moore,
C., Sprich, S., & Tsuang, M. T. (1992). Segregation analysis of attention
deficit hyperactivity disorder. Psychiatric Genetics, 2, 257-275.
Faraone, S. V., Biederman, J., Lehman, B., Keenan, K., Norman, D., Seidman,
L. J., Kolodny, R., Kraus, I., Perrin, J., & Chen, W. (1993). Evidence for
the independent familial transmission of attention deficit hyperactivity disorder
and learning disabilities: Results from a family genetic study. American Journal
of Psychiatry, 150, 891-895.
Faraone, S. V., Biederman, J., Mennin, D., Russell R., & Tsuang, M. T.
(1998). Familial subtypes of attention deficit hyperactivity disorder:
A 4-year follow-up study of children from antisocial-ADHD families. Journal
of Child Psychology and Psychiatry, 39, 1045-1053.
Faraone, S. V., Biederman, J., Mick, E., Williamson, S., Wilens, T., Spencer,
T., Weber, W., Jetton, J., Kraus, I., Pert, J., & Zallen, B. (2000).
Family study of girls with attention deficit hyperactivity disorder. American
Journal of Psychiatry, 157, 1077-1083.
Faraone, S. V., Biederman, J., & Monuteaux, M. C. (2001). Attention
deficit hyperactivity disorder with bipolar disorder in girls: further evidence
for a familial subtype? Journal of Affective Disorders, 64, 19-26.
Faraone, S. V., Biederman, J., Weber, W., & Russell R. L. (1998).
Psychiatric, neuropsychological, and psychosocial features of DSM-IV subtypes
of attention-deficit/hyperactivity disorder: Results from a clinically referred
sample. Journal of the American Academy of Child and Adolescent Psychiatry,37,
185-193.
Faraone, S. V., Biederman, J., Wozniak, J., Mundy, E., Mennin, D., & O’Donnell,
D. (1997). Is comorbidity with ADHD a marker for juvenile-onset mania?
Journal of the American Academy of Child and Adolescent Psychiatry, 36, 1046-1055.
Faraone, S. V., Lecendreaux, M., & Konofal, E. (2011). Growth dysregulation and ADHD: an epidemiological study of children in France. Journal of Attention Disorders, online first, DOI: 10.1177/1087054711413083.
Faraone, S. V., Bellgrove, M., Brikell. I., Cortese, S., Hartman, C., Hollis, C. Newcom, J., Philipsen, A., Polanczyk, G., Rubia, K., Sibley, M., Buitelaar, J. (2024). Attention deficit/hyperactivity disorder. Nature Reviews: Disease Primers, 10(Article 11), 1-21. doi.org/10.1038/s41572-024-00495-0
Fayyad, J., De Graaf, R., Kessler, R., Alonso, J., Angermeyer, M., Demyttenaere, K., De Girolamo, G., Haro, J., M., Karam, E. G., Lara, C., Lepine, J. P., Ormel, J., Pasada-Villa, J., Zaslavsky, A. M., & Jin, R. (2007). Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. British Journal of Psychiatry, 190, 402-409.
Fergusson, D. M., Fergusson, I. E., Horwood, L. J., & Kinzett, N. G. (1988).
A longitudinal study of dentine lead levels, intelligence, school performance,
and behaviour. Journal of Child Psychology and Psychiatry, 29, 811-824.
Fischer, M. (1990). Parenting stress and the child with attention deficit hyperactivity
disorder. Journal of Clinical Child Psychology, 19, 337-346.
Fischer, M., Barkley, R. A., Fletcher, K. & Smallish, L. (1993a). The stability
of dimensions of behavior in ADHD and normal children over an 8 year period.
Journal of Abnormal Child Psychology, 21, 315-337.
Fischer, M., Barkley, R. A., Fletcher, K., & Smallish, L. (1993b). The
adolescent outcome of hyperactive children diagnosed by research criteria, V:
Predictors of outcome. Journal of the American Academy of Child and Adolescent
Psychiatry, 32, 324-332.
Fischer, M., Barkley, R. A., Smallish, L., & Fletcher, K. R. (2004).
Hyperactive children as young adults: Deficits in attention, inhibition,
and response perseveration and their relationship to severity of childhood and
current ADHD and conduct disorder. Developmental Neuropsychology, 27,
107-113.
Fischer, M., Barkley, R. A., Smallish, L., & Fletcher, K. R. ().
Young adult outcome of hyperactive children: self-reported psychiatric disorders,
comorbidity, and the role of childhood conduct problems. Journal of Abnormal
Child Psychology, 30, 463-475.
Fletcher, K., Fischer, M., Barkley, R. A., & Smallish, L. (1996). A sequential
analysis of the mother-adolescent interactions of ADHD, ADHD/ODD, and
normal teenagers during neutral and conflict discussions. Journal of Abnormal
Child Psychology, 24, 271-298.
Frank, Y., Lazar, J. W., & Seiden, J. A. (1992). Cognitive event-related
potentials in learning-disabled children with or without attention-deficit hyperactivity
disorder. Annals of Neurology, 32, 478 (abstract).
Franke, B., Neale, B. M., & Faraone, S. V. (2009). Genome-wide association studies in ADHD. Human Genetics, online first, doi 10.1007/s00439-009-0663-4.
Frazier, T. W., Demareem H. A., & Youngstrom, E. A. (2004).
Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity
disorder. Neuropsychology, 18, 543-555.
Frick, P. J., Kamphaus, R. W., Lahey, B. B., Loeber, R., Christ, M. A. G.,
Hart, E. L., & Tannenbaum, L. E. (1991). Academic underachievement
and the disruptive behavior disorders. Journal of Consulting and Clinical
Psychology, 59, 289-294.
Froehlich, T. E., Anixt, J. S., Lee, I. M., Chirdkiatgumchai, V., Kuan, L., & Gilman, R. C. (2011). Update on environmental risk factors for attention deficit hyperactivity disorder. Current Psychiatry Reports, online first, doi 10.1007/s11920-011-0221-3.
Fuster, J. M. (1997). The prefrontal cortex (3rd ed.). New York: Raven Press.
Gadow, K. D., Nolan, E. E., Litcher, L., Carlson, G. A., Panina, N., Golovakha,
E., Sprafkin, J., & Bromet, E. J. (2000). Comparison of attention-deficit/hyperactivity
disorder symptom subtypes in Ukrainian schoolchildren. Journal of the
American Academy of Child and Adolescent Psychiatry,39, 1520-1527.
Gaub, M., & Carlson, C. L. (1997). Gender differences in ADHD: a
meta-analysis and critical review. Journal of the American Academy of
Child and Adolescent Psychiatry,36, 1036-1045.
Geller, B., & Luby, J. (1997). Child and adolescent bipolar disorder:
A review of the past 10 years. Journal of the American Academy of Child
and Adolescent Psychiatry, 36, 1168-1176.
Giedd, J. N., Castellanos, F. X., Casey, B. J., Kozuch, P., King, A. C., Hamburger,
S. D., & Rapoport, J. L. (1994). Quantitative morphology of the corpus
callosum in attention deficit hyperactivity disorder. American Journal
of Psychiatry, 151, 665-669.
Giedd, J. N., Snell, J. W., Lange, N., Rajapakse, J. C., Casey, B. J., Kozuch,
P. L., Vaituzis, A. C., Vauss, Y. C., Hamburger, S. D., Kaysen, D., & Rapoport,
J. L. (1996). Quantitative magnetic resonance imaging of human brain development:
Ages 4-18. Cerebral Cortex, 6, 551-560.
Gilger, J. W., Pennington, B. F., & DeFries, J. C. (1992). A twin study
of the etiology of comorbidity: Attention-deficit hyperactivity disorder and
dyslexia. Journal of the American Academy of Child and Adolescent Psychiatry,
31, 343-348.
Gillis, J. J., Gilger, J. W., Pennington, B. F., & Defries, J. C. (1992).
Attention deficit disorder in reading-disabled twins: Evidence for a genetic
etiology. Journal of Abnormal Child Psychology, 20, 303-315.
Gittelman, R., & Eskinazi, B. (1983). Lead and hyperactivity revisited.
Archives of General Psychiatry, 40, 827-833.
Gittelman, R., Mannuzza, S., Shenker, R., & Bonagura, N. (1985). Hyperactive
boys almost grown up: I. Psychiatric status. Archives of General Psychiatry,
42, 937-947.
Glow, P. H., & Glow, R. A. (1979). Hyperkinetic impulse disorder: A developmental
defect of motivation. Genetic Psychological Monographs, 100, 159-231.
Goldman-Rakic, P. S. (1987). Development of cortical circuitry and cognitive
function. Child Development, 58, 601-622.
Gomez, R., & Sanson, A. V. (1994). Mother-child interactions and
noncompliance in hyperactive boys with and without conduct problems. Journal
of Child Psychology and Psychiatry, 35, 477-490.
Grattan, L. M., & Eslinger, P. J. (1991). Frontal lobe damage in children
and adults: A comparative review. Developmental Neuropsychology, 7, 283-326.
Gray, J. A. (1982). The neuropsychology of anxiety. New York: Oxford University
Press.
Green, L., Fry, A. F., & Meyerson, J. (1994). Discounting of delayed rewards:
A life-span comparison. Psychological Science, 5, 33-36.
Grenell, M. M., Glass, C. R., & Katz, K. S. (1987). Hyperactive children
and peer interaction: Knowledge and performance of social skills. Journal
of Abnormal Child Psychology, 15, 1-13.
Gresham, F. M., MacMillan, d. L., Bocian, K. M., Ward, S. L., & Forness,
S. R. (1998). Comorbidity of hyperactivity-impulsivity-inattention and
conduct problems: risk factors in social, affective, and academic domains.
Journal of Abnormal Child Psychology, 26, 393-406.
Grodzinsky, G. M., & Diamond, R. (1992). Frontal lobe functioning in boys
with attention-deficit hyperactivity disorder. Developmental Neuropsychology,
8, 427-445.
Groen-Blokhuis, M. M., Middeldorp, C. M. Beljsterveldt, C. E. M. va, & Boomsma, D. I. (2012). Evidence for a causal association of low birth weight and attention problems. Journal of the America Academy of Child and Adolescent Psychiatry, 50, 1247-1254.
Gross-Tsur, V., Shalev, R. S., & Amir, N. (1991). Attention deficit disorder:
Association with familial-genetic factors. Pediatric Neurology, 7, 258-261.
Gruber, R., Sadeh, A., & Raviv, A. (2000). Instability of sleep patterns
in children with attention-deficit/hyperactivity disorder. Journal of
the American Academy of Child and Adolescent Psychiatry, 39, 495-501.
Gustafsson, P., Thernlund, G., Ryding, E., Rosen, I., & Cederblad, M. (2000).
Associations between cerebral blood-flow measured by single photon emission
computed tomorgraphy (SPECT), electro-encephalogram (EEG), behavior symptoms,
cognition and neurological soft signs in children with attention-deficit hyperactivity
disorder (ADHD). Acta Paediatrica, 89, 830-835.
Haenlein, M., & Caul, W. F. (1987). Attention deficit disorder with hyperactivity:
A specific hypothesis of reward dysfunction. Journal of the American Academy
of Child and Adolescent Psychiatry, 26, 356-362.
Halperin, J. M., & Gittelman, R. (1982). Do hyperactive children and their
siblings differ in IQ and academic achievement? Psychiatry Research, 6, 253-258.
Halperin, J. M., Newcorn, J. H., Koda, V. H., Pick, L., McKay, K. E., &
Knott, P. (1997). Noradrenergic mechanisms in ADHD children with and without
reading disabilities: A replication and extension. Journal of the American
Academy of Child and Adolescent Psychiatry, 36, 1688-1697.
Hamlett, K. W., Pellegrini, D. S., & Conners, C. K. (1987). An investigation
of executive processes in the problem solving of attention deficit disorder-hyperactive
children. Journal of Pediatric Psychology, 12, 227-240.
Hart, E. L., Lahey, B. B., Loeber, R., Applegate, B., & Frick, P. J. (1995).
Developmental changes in attention-deficit hyperactivity disorder in boys: A
four-year longitudinal study. Journal of Abnormal Child Psychology, 23,
729-750.
Hartsough, C. S., & Lambert, N. M. (1985). Medical factors in hyperactive
and normal children: Prenatal, developmental, and health history findings. American
Journal of Orthopsychiatry, 55, 190-210.
Harvey, W. J., & Reid, G. (1997). Motor performance of children with
attention-deficit hyperactivity disorder: A preliminary investigation.
Adapted Physical Activity Quarterly, 14, 189-202.
Hastings, J., & Barkley, R. A. (1978). A review of psychophysiological
research with hyperactive children. Journal of Abnormal Child Psychology, 7,
413-337.
Hauser, P., Zametkin, A. J., Martinez, P. et al. (1993). Attention deficit
hyperactivity disorder in people with generalized resistance to thyroid hormone.
New England Journal of Medicine, 328, 997-1001.
Heffron, W. A., Martin, C. A., & Welsh, R. J. (1984). Attention deficit
disorder in three pairs of monozygotic twins: A case report. Journal of the
American Academy of Child Psychiatry, 23, 299-301.
Hart, H., Radua, J., Nakao, T., Matatx-Cols, D., & Rubia, K. (2012). Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder. Archives of General Psychiatry, online first, doi:10.1001/jamapsychiatry.2013.277.
Hendren, R. L., De Backer, I., & Pandina, G. J. (2000). Review of
neuroimaging studies of child and adolescent psychiatric disorders from the
past 10 years. Journal of the American Academy of Child and Adolescent
Psychiatry, 39, 815-828.
Henry, B., Moffitt, T. E., Caspi A., Langley, J., & Silva, P. A. (1994).
On the “remembrance of things past”: A longitudinal evaluation of
the retrospective method. Psychological Assessment, 6, 92-101.
Herpertz, S. C., Wenning, B., Mueller, B., Qunaibi, M., Sass, H., & Herpetz-Dahlmann,
B. (2001). Psychological responses in ADHD boys with and without conduct
disorder: Implications for adult antisocial behavior. Journal of the American
Academy of Child and Adolescent Psychiatry, 40, 1222-1230.
Hervey, A. S., Epstein, J. N., & Curry, J. F. (2004). Neuropsychology
of adults with attention-deficit/hyperactivity disorder: A meta-analytic review.
Neuropsychology, 18, 495-503.
Hinshaw, S. P. (1987). On the distinction between attentional deficits/hyperactivity
and conduct problems/aggression in child psychopathology. Psychological
Bulletin, 101, 443-447.
Hinshaw, S. P. (1992). Externalizing behavior problems and academic underachievement
in childhood and adolescence: Causal relationships and underlying mechanisms.
Psychological Bulletin, 111, 127-155.
Hinshaw, S. P. (1994). Attention deficits and hyperactivity in children. Thousand
Oaks, CA: Sage.
Hinshaw, S. P. (2001). Is the inattentive type of ADHD a separate disorder?
Clinical Psychology: Science and Practice, 8, 498-501.
Hinshaw, S. P., Heller, T., & McHale, J. P. (1992). Covert antisocial behavior
in boys with attention-deficit hyperactivity disorder: External validation and
effects of methylphenidate. Journal of Consulting and Clinical Psychology, 60,
274-281.
Hinshaw, S. P., Herbsman, C., Melnick, S., Nigg, J., & Simmel, C. (1993,
February). Psychological and familial processes in ADHD: Continuous or discontinuous
with those in normal comparison children? Paper presented at the Society for
Research in Child and Adolescent Psychopathology, Santa Fe, NM.
Hinshaw, S. P., & Melnick, S. M. (1995). Peer relationships in boys
with attention-deficit hyperactivity disorder with and without comorbid aggression.
Development and Psychopathology, 7, 627-647.
Hinshaw, S. P., Morrison, D. C., Carte, E. T., & Cornsweet, C. (1987).
Factorial dimensions of the Revised Behavior Problem Checklist: Replication
and validation within a kindergarten sample. Journal of Abnormal Child Psychology,
15, 309-327.
Hodgens, J. B., Cole, J., & Boldizar, J. (2000). Peer-based differences
among boys with ADHD. Journal of Clinical Child Psychology, 29, 443-452.
Hohman, L. B. (1922). Post-encephalitic behavior disorders in children. Johns
Hopkins Hospital Bulletin, 33, 372-375.
Holdsworth, L., & Whitmore, K. (1974). A study of children with epilepsy
attending ordinary schools: I. Their seizure patterns, progress, and behaviour
in school. Developmental Medicine and Child Neurology, 16, 746-758.
Hoy, E., Weiss, G., Minde, K., & Cohen, N. (1978). The hyperactive
child at adolescence: Cognitive, emotional, and social functioning. Journal
of Abnormal Child Psychology, 6, 311-324.
Hoza, B. (2007). Peer functioning in children with ADHD. Journal of Pediatric Psychology, 32, 655-661.
Hoza, B., Pelham, W. E., Waschbusch, D. A., Kipp, H., & Owens, J. S. (2001).
Academic task performance of normally achieving ADHD and control boys: performance,
self-evaluations, and attributions. Journal of Consulting and Clinical
Psychology, 69, 271-283.
Humphries, T., Kinsbourne, M., & Swanson, J. (1978). Stimulant effects
on cooperation and social interaction between hyperactive children and their
mothers. Journal of Child Psychology and Psychiatry, 19, 13-22.
Humphries, T., Koltun, H., Malone, M., & Roberts, W. (1994). Teacher-identified
oral language difficulties among boys with attention problems. Developmental
and Behavioral Pediatrics, 15, 92-98.
Jacobvitz, D., & Sroufe, L. A. (1987). The early caregiver-child
relationship and attention-deficit disorder with hyperactivity in kindergarten:
A prospective study. Child Development, 58, 1488-1495.
James, W. (1890). The principles of psychology. London: Dover.
Jensen, P. S., Martin, D., & Cantwell, D. P. (1997). Comorbidity
in ADHD: Implications for research, practice, and DSM-V. Journal of the
American Academy of Child and Adolescent Psychiatry, 36, 1065-1079.
Jensen, P. S., Shervette, R. E., Xenakis, S. N., & Bain, M. W. (1988).
Psychosocial and medical histories of stimulant-treated children. Journal
of the American Academy of Child and Adolescent Psychiatry, 27, 798-801.
Jensen, P. S., Shervette, R. E. III, Xenakis, S. N., & Richters, J. (1993).
Anxiety and depressive disorders in attention deficit disorder with hyperactivity:
New Findings. American Journal of Psychiatry, 150, 1203-1209.
Jensen, P. S., Watanabe, H. K., Richters, J. E., Cortes, R., Roper, M., &
Liu, S. (1995). Prevalence of mental disorder in military children and
adolescents: Findings from a two-stage community survey. Journal of the
American Academy of Child and Adolescent Psychiatry, 34, 1514-1524.
Johnson, J. G., Cohen, P., Kasen, S., Smailes, E., & Brook, J. S. (2001).
Association of maladaptive parental behavior with psychiatric disorder among
parents and their offspring. Archives of General Psychiatry, 58, 453-460.
Johnson, R. C., & Rosen, L. A. (2000). Sports behavior of ADHD children.
Journal of Attention Disorders, 4, 150-160.
Johnston, C. (1996). Parent characteristics and parent-child interactions
in families of nonproblem children and ADHD children with higher and lower levels
of oppositional-defiant disorder. Journal of Abnormal Child Psychology,
24, 85-104.
Johnston, C., & Mash, E. J. (2001). Families of children with attention-deficit/hyperactivity
disorder: Review and recommendations for future research. Clinical Child
and Family Psychology Review, 4, 183-207.
Johnston, C., Mash, E. J., Miller, N., & Ninowski, J. F. (2012). Parnting in adults with attention-deficit/hyperactivity disorder (ADHD). Clinical Psychology Review, 32, 215-231.
Johnstone, S. J., Barry, R. J., & Anderson, J. W. (2001). Topographic
distribution and developmental timecourse of auditory event-related potentials
in two subtypes of attention-deficit hyperactivity disorder. International
Journal of Psychophysiology, 42, 73-94.
Kadesjo, B., & Gillberg, C. (2001). The comorbidity of ADHD in the
general population of Swedish school-age children. Journal of Child Psychology
and Psychiatry, 42, 487-492.
Kanbayashi, Y., Nakata, Y., Fujii, K., Kita, M., & Wada, K. (1994). ADHD-related
behavior among non-referred children: Parents’ ratings of DSM-III-R symptoms.
Child Psychiatry and Human Development, 25, 13-29.
Kanfer, F. H., & Karoly, P. (1972). Self-control: A behavioristic excursion
into the lion’s den. Behavior Therapy, 3, 398-416.
Kaplan, B. J., McNichol, J., Conte, R. A., & Moghadam, H. K. (1987). Sleep
disturbance in preschool-aged hyperactive and nonhyperactive children. Pediatrics,
80, 839-844.
Keenan, K. (2000). Emotion dysregulation as a risk factor for child psychopathology.
Clinical Psychology: Science and Practice, 7, 418-434.
Kessler, J. W. (1980). History of minimal brain dysfunction. In H. Rie &
E. Rie (Eds.), Handbook of minimal brain dysfunctions: A critical view (pp.
18-52). New York: Wiley.
Kessler, R. C., Berglund, P., Demler, O., Jin, R., & Walters, E. E. (2005).
Lifetime prevalence and age-of-onset distribution of DSM-IV disorders in the
national comorbidity survey replication. Archives of General Psychiatry,
62, 593-602.
Klein, R.,G., Mannuzza, S., Olaagastt, M. A. R., Rotzen, E., Hutchison, J. A., Lashua, E. C., & Castellanos, F. X. (2012). Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Archives of General Psychiatry, online first, doi:10.1001 /archgenpsychiatry. 2012.271.
Klorman, R. (1992). Cognitive event-related potentials in attention deficit
disorder. In S. E. Shaywitz & B. A. Shaywitz (Eds.), Attention deficit disorder
comes of age: Toward the twenty-first century (pp. 221-244). Austin, TX:
Pro-ed.
Klorman, R., Salzman, L. F., & Borgstedt, A. D. (1988). Brain event-related
potentials in evaluation of cognitive deficits in attention deficit disorder
and outcome of stimulant therapy. In L. Bloomingdale (Ed.), Attention deficit
disorder (Vol. 3, pp. 49-80). New York: Pergamon.
Klorman, R., Hazel-Fernandez, H., Shaywitz, S. E., Fletcher, J. M., Marchione,
K. E., Holahan, J. M., Stuebing, K. K., & Shaywitz, B. A. (1999).
Executive functioning deficits in attention-deficit/hyperactivity disorder are
independent of oppositional defiant or reading disorder. Journal of the
American Academy of Child and Adolescent Psychiatry, 38, 1148-1155.
Knobel, M., Wolman, M. B., & Mason, E. (1959). Hyperkinesis and organicity
in children. Archives of General Psychiatry, 1, 310-321.
Kohn, A. (1989, November). Suffer the restless children. The Atlantic Monthly,
pp. 90-100.
Kopp, C. B. (1982). Antecedents of self-regulation: A developmental perspective.
Developmental Psychology, 18, 199-214.
Kroes, M., Kalff, A. C., Kessels, A. G. H., Steyaert, J., Feron, F., can Someren,
A., Hurks, P., Hendriksen, J., can Zeban, T., Rozendaal, N., Crolla, I., Troost,
J., Jolles, J., & Vles, J. (2001). Child psychiatric diagnoses in
a population of Dutch schoolchildren aged 6 to 8 years. Journal of the
American Academy of Child and Adolescent Psychiatry,40, 1401-1409.
Kuntsi, J., Oosterlaan, J., & Stevenson, J. (2001). Psychological
mechanisms in hyperactivity: I. Response inhibition deficit, working memory
impairment, delay aversion, or something else? Journal of Child Psychology
and Psychiatry, 42, 199-210.
Lahey, B. B. (2001). Should the Combined and Predominantly Inattentive
Types of ADHD be considered distinct and unrelated disorders? Not now,
at least. Clinical Psychology: Science and Practice, 8, 494-497.
Lahey, B. B., Applegate, B., McBurnett, K., Biederman, J., Greenhill, L., et
al. (1994). DSM-IV field trials for attention deficit/hyperactivity disorder
in children and adolescents. American Journal of Psychiatry, 151, 1673-1685.
Lahey, B. B., McBurnett, K., & Loeber, R. (2000). Are attention-deficit/hyperactivity
disorder and oppositional defiant disorder developmental precursors to conduct
disorder? In A. J. Sameroff, M. Lewis, & S. M. Miller (Eds.), Handbook
of developmental psychopathology (2nd ed.) (pp. 431-446.). New York: Plenum.
Lahey, B. B., Pelham, W. E., Schaughency, E. A., Atkins, M. S., Murphy, H.
A., Hynd, G. W., Russo, M., Hartdagen, S., & Lorys-Vernon, A. (1988). Dimensions
and types of attention deficit disorder with hyperactivity in children: A factor
and cluster-analytic approach. Journal of the American Academy of Child and
Adolescent Psychiatry, 27, 330-335.
Lambert, N. M. (1988). Adolescent outcomes for hyperactive children. American
Psychologist, 43, 786-799.
Lambert, N. M., Hartsough, C. S. (1998). Prospective study of tobacco
smoking and substance dependencies among samples of ADHD and non-ADHD participants.
Journal of Learning Disabilities, 31, 533-544
Lambert NM. Stimulant treatment as a risk factor for nicotine use and
substance abuse. In PS Jensen, JR Cooper, eds., Diagnosis and Treatment
of Attention Deficit Hyperactivity Disorder: An Evidence-Based Approach.
New York: American Medical Association Press, in press
Lambert, N. M., Sandoval, J., & Sassone, D. (1978). Prevalence of hyperactivity
in elementary school children as a function of social system definers. American
Journal of Orthopsychiatry, 48, 446-463.
Lamminmaki, T., Ahonen, T., Narhi, V., Lyytinent, H., & de Barra, H. T.
(1995). Attention deficit hyperactivity disorder subtypes: Are there differences
in academic problems? Developmental Neuropsychology, 11, 297-310.
Landau, S., Berk, L. E., & Mangione, C. (1996, March). Private speech
as a problem-solving strategy in the face of academic challenge: The failure
of impulsive children to get their act together. Paper presented at the
meeting of the National Association of School Psychologists, Atlanta, GA.
Lang, P. (1995). The emotion probe. American Psychologist, 50I,
372-385.
Langberg, J. M., Molina, B. S. G., Arnold, L. E., Epstein, J. N., & Altaye, M. (2011). Patterns and predictors of adolescent academic achievement and performance in a sample of children with attention-deficit/hyperactivity disorder. Journal of Clinical Child and Adolescent Psychology, 40, 1-13.
Langsdorf, R., Anderson, R. F., Walchter, D., Madrigal, J. F., & Juarez,
L. J. (1979). Ethnicity, social class, and perception of hyperactivity. Psychology
in the Schools, 16, 293-298.
Lapouse, R., & Monk, M. (1958). An epidemiological study of behavior characteristics
in children. American Journal of Public Health, 48, 1134-1144.
Last, C. G., Hersen, M., Kazdin, A., Orvaschel, H., & Perrin, S. (1991).
Anxiety disorders in children and their families. Archives of General Psychiatry,
48, 928-934.
Laufer, M., Denhoff, E., & Solomons, G. (1957). Hyperkinetic impulse disorder
in children’s behavior problems. Psychosomatic Medicine, 19, 38-49.
Lavigne, J. V., Gibbons, R. D., Christoffel,. K., Arend, R., Rosenbaum, D.,
Binns, H., Dawson, N., Sobel, H., & Isaacs, C. (1996). Prevalence
rates and correlates of psychiatric disorders among preschool children.
Journal of the American Academy of Child and Adolescent Psychiatry,35, 204-214.
Lecendreux, M., Konofal, E., Bouvard, M., Falissard, B., Simeoni, M. M. (2000).
Sleep and alertness in children with ADHD. Journal of Child Psychology
and Psychiatry, 41, 803-812.
Lerner, J. A., Inui, T. S., Trupin, E. W., & Douglas, E. (1985). Preschool
behavior can predict future psychiatric disorders. Journal of the American Academy
of Child Psychiatry, 24, 42-48.
Levin, P. M. (1938). Restlessness in children. Archives of Neurology and Psychiatry,
39, 764-770.
Levy, F., & Hobbes, G. (1989). Reading, spelling, and vigilance in attention
deficit and conduct disorder. Journal of Abnormal Child Psychology, 17, 291-298.
Lingineni, R., Biswas, S., Ahmad, N., Jackson, B. E., Bae, S., & Singh, K. P. (2012). Factors association with attention deficit/hyperactivity disorder among US children: Results from a national survey. BMC Pediatrics, 12:50, online first, doi:10.1186/1471-2431-12-50.
Liu, X., Kurita, H., Guo, C., Tachimori, H., Ze, J., & Okawa, M. (2000).
Behavioral and emotional problems in Chinese children: teacher reports for ages
6 to 11. Journal of Child Psychology and Psychiatry, 41, 253-260.
Loe, I. M., & Feldman, H. M. (2007). Academic and educational outcomes of children with ADHD. Journal of Pediatric Psychology, 32, 643-654.
Loeber, R., Burke, J. D., Lahey, B. B., Winters, A., & Zera, M. (2000).
Oppositional defiant and conduct disorder: A review of the past 10 years, Part
I. Journal of the American Academy of Child and Adolescent Psychiatry,
39, 1468-1484.
Loeber, R., Green, S. M., Lahey, B. B., Christ, M. A. G., & Frick, P. J.
(1992). Developmental sequences in the age of onset of disruptive child behaviors.
Journal of Child and Family Studies, 1, 21-41.
Loney, J., Kramer, J., & Milich, R. (1981). The hyperkinetic child grows
up: Predictors of symptoms, delinquency, and achievement at follow-up. In K.
Gadow & J. Loney (Eds.), Psychosocial aspects of drug treatment for hyperactivity.
Boulder, CO: Westview Press.
Loney, J., Kramer, J. R., & Salisbury, H. (in press). Medicated versus
unmedicated ADHD children: adult involvement with legal and illegal drugs.
In PS Jensen, JR Cooper, eds., Diagnosis and Treatment of Attention Deficit
Hyperactivity Disorder: An Evidence-Based Approach. New York: American
Medical Association Press.
Loo, S. & Barkley, R. A. (2005). Clinical utility of EEG in attention
deficit hyperactivity disorder. Applied Neuropsychology, 12, 64-76.
Loo, S. K., & Makeig, S., (2012). Clinical utility of EEG in attention-deficit hyperactivity disorder: a research update. Neurotherapeutics, 9, 569-587.
Lorch, E. P., Milich, M., Sanchez, R. P., van den Broek, P., Baer, S., Hooks,
K., Hartung, C., & Welsh, R. (2000). Comprehension of televised stories
in boys with attention deficit/hyperactivity disorder and nonreferred boys.
Journal of Abnormal Psychology, 109, 321-330.
Luk, S. (1985). Direct observations studies of hyperactive behaviors. Journal
of the American Academy of Child and Adolescent Psychiatry, 24, 338-344.
Lynam, D., Moffitt, T., & Stouthamer-Loeber, M. (1993). Explaining the
relation between IQ and delinquency: Class, race, test motivation, school failure,
or self-control? Journal of Abnormal Psychology, 102, 187-196.
Lyon, G. R. (1995). Attention, memory, and executive functions. Baltimore:
Brookes.
Madan-Swain, A., & Zentall, S. S. (1990). Behavioral comparisons
of liked and disliked hyperactive children in play contexts and the behavioral
accommodations by their classmates. Journal of Consulting and Clinical
Psychology, 58, 197-209.
Maedgen, J. W., & Carlson, C. L. (2000). Social functioning and emotional
regulation in the attention deficit hyperactivity disorder subtypes. Journal
of Clinical Child Psychology, 29, 30-42.
Malone, M. A., & Swanson, J. M. (1993). Effects of methylphenidate on impulsive
responding in children with attention deficit hyperactivity disorder. Journal
of Child Neurology, 8, 157-163.
Mannuzza, S., & Gittelman, R. (1986). Informant variance in the diagnostic
assessment of hyperactive children as young adults. In J. E. Barrett &
R. M. Rose (Eds.), Mental disorders in the Community (pp. 243-254). New
York: Guilford.
Mannuzza, S., Klein, R., Bessler, A., Malloy, P., & LaPadula, M. (1993).
Adult outcome of hyperactive boys: Educational achievement, occupational rank,
and psychiatric status. Archives of General Psychiatry, 50, 565-576.
Mannuzza, S., Klein, R., Bessler, A., Malloy, P., & LaPadula, M. (1998).
Adult psychiatric status of hyperactive boys grown up. American Journal
of Psychiatry, 155, 493-498.
Mannuzza, S., Klein, R. G., Bonagura, N., Malloy, P., Giampino, H., & Addalli,
K. A. (1991). Hyperactive boys almost grown up: replication of psychiatric
status. Archives of General Psychiatry, 48, 77-83.
Mannuzza, S., & Klein, R. (1992). Predictors of outcome of children with
attention-deficit hyperactivity disorder. In G. Weiss (Ed.), Child and adolescent
psychiatric clinics of North America: Attention-deficit hyperactivity disorder
(pp. 567-578). Philadelphia: Saunders.
Mariani, M., & Barkley, R. A. (1997). Neuropsychological and academic
functioning in preschool children with attention deficit hyperactivity disorder.
Developmental Neuropsychology, 13, 111-129.
Marshall, R. M., Hynd, G. W., Handwerk, M. J. et al., (1997). Academic
underachievement in ADHD subtypes. Journal of Learning Disabilities, 30,
635-642.
Mash, E. J., & Johnston, C. (1982). A comparison of mother- child
interactions of younger and older hyperactive and normal children. Child Development,
53, 1371-1381.
Mash, E. J., & Johnston, C. (1983a). Sibling interactions of hyperactive
and normal children and their relationship to reports of maternal stress and
self-esteem. Journal of Clinical Child Psychology, 12, 91-99.
Mash, E. J., & Johnston, C. (1983b). The prediction of mothers’ behavior
with their hyperactive children during play and task situations. Child and Family
Behavior Therapy, 5, 1-14.
Mash, E. J., & Johnston, C. (1990). Determinants of parenting stress: Illustrations
from families of hyperactive children and families of physically abused children.
Journal of Clinical Child Psychology, 19, 313-328.
Mattes, J. A. (1980). The role of frontal lobe dysfunction in childhood hyperkinesis.
Comprehensive Psychiatry, 21, 358-369.
Matthys, W., Cuperus, J. M., & Van Engeland, H. (1999). Deficient
social problem-solving in boys with ODD/CD, with ADHD, and with both disorders.
Journal of the American Academy of Child and Adolescent Psychiatry,38, 311-321.
Matthys, W., van Goozen, S. H. M., de Vries, H., Cohen-Kettenis, P. T., &
van Engeland, H. (1998). The dominance of behavioural activation over
behavioural inhibition in conduct disordered boys with or without attention
deficit hyperactivity disorder. Journal of Child Psychology and Psychiatry,
39, 643-651.
McArdle, P., O’Brien, G., & Kolvin, I. (1995). Hyperactivity: Prevalence
and relationship with conduct disorder. Journal of Child Psychology and Psychiatry,
36, 279-303.
McBurnett, K., Pfiffner, L. J., & Frick, P. J. (2001). Symptom properties
as a function of ADHD type: an argument for continued study of sluggish cognitive
tempo. Journal of Abnormal Child Psychology, 29, 207-213.
McGee, R., Stanton, W. R., & Sears, M. R. (1993). Allergic disorders and
attention deficit disorder in children. Journal of Abnormal Child Psychology,
21, 79-88.
McGee, R., Williams, S., & Feehan, M. (1992). Attention deficit disorder
and age of onset of problem behaviors. Journal of Abnormal Child Psychology,
20, 487-502.
McGee, R., Williams, S., & Silva, P. A. (1984). Behavioral and developmental
characteristics of aggressive, hyperactive, and aggressive-hyperactive
boys. Journal of the American Academy of Child Psychiatry, 23, 270-279.
McLoughlin, G., Ronald, A., Kuntsi, J., Asherson, P., & Plomin, R. (2007).
Genetic support for the dual nature of attention deficit hyperactivity disorder:
substantial genetic overlap between the inattentive and hyperactive-impulsive
components. Journal of Abnormal Child Psychology, 35, 999-1008.
McMahon, S. A., & Greenberg, L. M. (1977). Serial neurologic examination
of hyperactive children. Pediatrics, 59, 584-587.
Meachon, E. J., Schaider, J. P., & Alpers, G. W. (2025). Motor skills in children with ADHD: Overlap with developmental coordination disorder. BMC Psychology, 13 (Article 53). doi:10.1186/s40359-024-02282-8
Melnick, S. M., & Hinshaw, S. P. (1996). What they want and what
they get: the social goals of boys with ADHD and comparison boys. Journal
of Abnormal Child Psychology, 24, 169-185.
Melnick, S. M., & Hinshaw, S. P. (2000). Emotion regulation and parenting
in AD/HD and comparison boys: Linkages with social behaviors and peer preference.
Journal of Abnormal Child Psychology, 28, 73-86.
Michon, J. (1985). Introduction. In J. Michon & T. Jackson (Eds.), Time,
mind, and behavior. Berlin: Springer-Verlag.
Milberger, S. (1997). Impact of adversity on functioning and comorbidity
in girls with ADHD. Paper presented at the annual meeting of the American
Academy of Child and Adolescent Psychiatry, Toronto, October.
Milberger, S., Biederman, J., Faraone, S. V., Chen, L., & Jones, J. (1996b).
ADHD is associated with early initiation of cigarette smoking in children and
adolescents. Journal of the American Academy of Child and Adolescent Psychiatry,
36, 37-44.
Milich, R., Hartung, C. M., Matrin, C. A., & Haigler, E. D. (1994). Behavioral
disinhibition and underlying processes in adolescents with disruptive behavior
disorders. In D. K. Routh (Ed.), Disruptive behavior disorders in childhood
(pp. 109-138). New York: Plenum Press.
Milich, R., Ballentine, A. C., & Lynam, D. (2001). ADHD Combined
Type and ADHD Predominantly Inattentive Type are distinct and unrelated disorders.
Clinical Psychology: Science and Practice, 8, 463-488.
Minde, K., Webb, G., & Sykes, D. (1968). Studies on the hyperactive
child, VI: Prenatal and perinatal factors associated with hyperactivity.
Developmental Medicine and Child Neurology, 10, 355-363.
Mirsky, A. F. (1996). Disorders of attention: A neuropsychological perspective.
In R. G. Lyon & N. A. Krasnegor (Eds.), Attention, memory, and executive
function (pp. 71-96). Baltimore, MD: Paul H. Brookes.
Mitchell, E. A., Aman, M. G., Turbott, S. H., & Manku, M. (1987). Clinical
characteristics and serum essential fatty acid levels in hyperactive children.
Clinical Pediatrics, 26, 406-411.
Mitsis, E. M., McKay, K. E., Schulz, K. P., Newcorn, J. H., & Halperin,
J. M. (2000). Parent-teacher concordance in DSM-IV attention-deficit/hyperactivity
disorder in a clinic-referred sample. Journal of the American Academy of
Child and Adolescent Psychiatry, 39, 308-313.
Moffitt, T. E. (1990). Juvenile delinquency and attention deficit disorder:
Boys’ developmental trajectories from age 3 to 15. Child Development,
61, 893-910.
Molina, B. S. G., & Pelham, W. E. (2001). Substance use, substance
abuse, and LD among adolescents with a childhood history of ADHD. Journal
of Learning Disabilities,, 333-342.
Molina, B. S. G., Smith, B. H., & Pelham, W. E. (1999). Interactive effects
of attention deficit hyperactivity disorder and conduct disorder on early adolescent
substance use. Psychology of Addictive Behavior, 13, 348-358
Mori, L., & Peterson, L. (1995). Knowledge of safety of high and
low active-impulsive boys: Implications for child injury prevention. Journal
of Clinical Child Psychology, 24, 370-376.
Morgan, A. E., Hynd, G. W., Riccio, C. A., & Hall, J. (1996). Validity
of DSM-IV predominantly inattentive and combined types: relationship to previous
DSM diagnoses/subtype differences. Journal of the American Academy of
Child and Adolescent Psychiatry,35, 325-333.
Morrison, J., & Stewart, M. (1973). The psychiatric status of the legal
families of adopted hyperactive children. Archives of General Psychiatry, 28,
888-891.
Murphy, K. R., Barkley, R. A., Bush, T. (2001). Executive functioning and olfactory
identification in young adults with attention deficit hyperactivity disorder.
Neuropsychology, 15, 211-220.
Nada-Raja, S., Langley, J. D., McGee, R., Williams, S. M., Begg, D. J., &
Reeder, A. I. (1997). Inattentive and hyperactive behaviors and driving
offenses in adolescence. Journal of the American Academy of Child and Adolescent
Psychiatry, 36, 515-522.
Needleman, H. L., Gunnoe, C., Leviton, A., Reed, R., Peresie, H., Maher, C.,
& Barrett, P. (1979). Deficits in psychologic and classroom performance
of children with elevated dentine lead levels. New England Journal of Medicine,
300, 689-695.
Needleman, H. L., Schell, A. Bellinger, D. C., Leviton, L., & Alfred, E.
D. (1990). The long-term effects of exposure to low doses of lead in childhood: An 11-year follow-up report. New England Journal of Medicine,
322, 83-88.
Neuman, R. J., Lobos, E., Reich, W., Henderson, C. A., Sun, L., & Todd, R. D. (2007). Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biological Psychiatry, 61, 1320-1328.
Newcorn, J. H., Halperin, J. M., Jensen, P. S., Abikoff, H. B., Arnold, L.
E., Cantwell, D. P., Conners, C. K., Elliott, G. R., Epstein, J. N., Greenhill,
L. L., Hechtman, L., Hinshaw, S. P., Hoza, B., Kraemer, H. C., Pelham, W. E.,
Severe, J. B., Swanson, J. M., Wells, K. C., Wigal, T., & Vitiello, B. (2001).
Symptom profiles in children with ADHD: Comorbidity and gender. Journal
of the American Academy of Child and Adolescent Psychiatry, 40, 137-146.
Nigg, J. T. (1999). The ADHD response-inhibition deficit as measured
by the stop task: Replication with DSM-IV Combined Type, extension, and qualification.
Journal of Abnormal Child Psychology, 27, 393-402.
Nigg, J. T. (2000). On inhibition/disinhibition in developmental psychopathology:
Views from cognitive and personality psychology and a working inhibition taxonomy.
Psychological Bulletin, 126, 220-246.
Nigg, J. T. (2001). Is ADHD an inhibitory disorder? Psychological
Bulletin, 125, 571-596.
Nigg, J. T. (2006). What Causes ADHD? New York: Guilford Publications.
Nigg, J. T., & Casey, B. J. (2005). An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Development and Psychopathology, 17, 765-806.
Nijmeijer, J. S., Minderaa, R. B., Buitelaar, J. K., Mulligan, A., Hartman, C. A., & Hoekstra, P. J. (2008). Attention-deficit/hyperactivity disorder and social dysfunctioning. Clinical Psychology Review, 28, 692-708.
Nigg, J. T., Hinshaw, S. P., Carte, E. T., & Treuting, J. J. (1998).
Neuropsychological correlates of childhood attention-deficit/hyperactivity disorder:
Explainable by comorbid disruptive behavior or reading problems? Journal
of Abnormal Psychology, 107, 468-480.
Nikolas, M. A., & Burt, S. A. (2010). Genetic and environmental influences on ADHD symptom dimensions of inattention and hyperactivity: a meta-analysis. Journal of Abnormal Psychology, 119, 1-17.
Nucci, L. P., & Herman, S. (1982). Behavioral disordered children’s conceptions of moral, conventional, and personal issues. Journal of Abnormal Child Psychology, 10, 411-426.
O’Connor, M., Foch, T., Sherry, T., & Plomin, R. (1980). A twin study
of specific behavioral problems of socialization as viewed by parents. Journal
of Abnormal Child Psychology, 8, 189-199.
O’Dougherty, M., Nuechterlein, K. H., & Drew, B. (1984). Hyperactive
and hypoxic children: Signal detection, sustained attention, and behavior.
Journal of Abnormal Psychology, 93, 178-191.
O’Leary, K. D., Vivian, D., & Nisi, A. (1985). Hyperactivity in Italy.
Journal of Abnormal Child Psychology, 13, 485-500.
Olson, S. L., Bates, J. E., Sandy, J. M., & Lanthier, R. (2000).
Early developmental precursors of externalizing behavior in middle childhood
and adolescence. Journal of Abnormal Child Psychology, 28, 119-133.
Olson, S. L., Schilling, E. M., & Bates, J. E. (1999). Measurement
of impulsivity: Construct coherence, longitudinal stability, and relationship
with externalizing problems in middle childhood and adolescence. Journal
of Abnormal Child Psychology, 27, 151-165.
O’Nions, E. et al. (2025). Life expectancy and years of life lost for adults diagnosed with ADHD in the UK: matched cohort study. British Journal of Psychiatry (Pub.online). doi:10.1192/bjp.2024.199
Oosterlaan, J., Logan, G. D., & Sergeant, J. A. (1998). Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: A meta-analysis of studies with the Stop Task. Journal of Child Psychology and Psychiatry, 39, 411-425.
Overgaard, K. R., Oerbeck, B., Friis, S., Pripp, A.H., Aase, H., Ingeborgrud, C.B., Biele, G. (2024). Functional impairment related to ADHD from preschool to school age. Journal of Attention Disorders, 29(3): 220-230. doi.org/10.1177/10870547241301179
Palfrey, J. S., Levine, M. D., Walker, D. K., & Sullivan, M. (1985). The
emergence of attention deficits in early childhood: A prospective study. Developmental
and Behavioral Pediatrics, 6, 339-348.
Palmer, E. D. & Finger, S. (2001). An early description of ADHD (Inattentive Subtype): Dr. Alexander Crichton and "Menal Restlessness" (1798). Child Psychology and Psychiatry Review, 6, 66-73.
Parry, P. A., & Douglas, V. I. (1983). Effects of reinforcement on concept
identification in hyperactive children. Journal of Abnormal Child Psychology,
11, 327-340.
Patterson, G. R., Degarmo, D. S., & Knutson, N. (2000). Hyperactive and
antisocial behaviors: Comorbid or two points in the same process. Development
& Psychopathology, 12, 91-106.
Pauls, D. L. (1991). Genetic factors in the expression of attention-deficit
hyperactivity disorder. Journal of Child and Adolescent Psychopharmacology,
1, 353-360.
Pauls, D. L., Hurst, C. R., Kidd, K. K., Kruger, S. D., Leckman, J. F., &
Cohen, D. J. (1986). Tourette Syndrome and attention deficit disorder: Evidence
against a genetic relationship. Archives of General Psychiatry, 43, 1177-
1179.
Pelham, W. E. Jr. (2001). Are ADHD/I and ADHD/C the same or different?
Does it matter? Clinical Psychology: Science and Practice, 8, 502-506.
Pelham, W. E., & Lang, A. R. (1993). Parental alcohol consumption and deviant
child behavior: Laboratory studies of reciprocal effects. Clinical Psychology
Review, 13, 763-784.
Pennington, B. F., & Ozonoff, S. (1996). Executive functions and
developmental psychopathology. Journal of Child Psychology and Psychiatry,
37, 51-87.
Penny, A. M., Waschbusch, D. A., Klein, R. M., Corkum, P., & Eskes, G. (2009). Developing a measure of sluggish cognitive tempo for children: Content validity, factor structure, and reliability. Psychological Assessment, 21, 380-389.
Peterson, B. S., Pine, D. S., Cohen, P., & Brook, J. S. (2001). Prospective,
longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity
disorders in an epidemiological sample. Journal of the American Academy
of Child and Adolescent Psychiatry, 40, 685-695.
Pfiffner, L. J., McBurnett, K., & Rathouz, P. J. (2001). Father absence
and familial antisocial characteristics. Journal of Abnormal Child Psychology,
29, 357-367.
Pike, A., & Plomin, R. (1996). Importance of nonshared environmental
factors for childhood and adolescent psychopathology. Journal of the American
Academy of Child and Adolescent Psychiatry, 35, 560-570.
Pillow, D. R., Pelham, W. E., Jr., Hoza, B., Molina, B. S. G., & Stultz,
C. H. (1998). Confirmatory factor analyses examining attention deficit
hyperactivity disorder symptoms and other childhood disruptive behaviors.
Journal of Abnormal Child Psychology, 26, 293-309.
Pineda, D., Ardila, A., Rosselli, M., Arias, B. E., Henao, G. C., Gomex, L.
F., Mejia, S. E., & Miranda, M. L. (1999). Prevalence of attention-deficit/hyperactivity
disorder symptoms in 4- to 17-year old children in the general population.
Journal of Abnormal Child Psychology, 27, 455-462.
Pliszka, S. R. (1992). Comorbidity of attention-deficit hyperactivity disorder
and overanxious disorder. Journal of the American Academy of Child and Adolescent
Psychiatry, 31, 197-203.
Pliszka, S. R., Liotti, M., & Woldorff, M. G. (2000). Inhibitory
control in children with attention-deficit/hyperactivity disorder: Event-related
potentials identify the processing component and timing of an impaired right-frontal
response-inhibition mechanism. Biological Psychiatry, 48, 238-246.
Pliszka, S. R., McCracken, J. T., & Maas, J. W. (1996). Catecholamines
in attention deficit hyperactivity disorder: current perspectives. Journal
of the American Academy of Child and Adolescent Psychiatry, 35, 264-272.
Plomin, R. (1995). Genetics and children’s experiences in the family.
Journal of Child Psychology and Psychiatry, 36, 33-68.
Plomin, R., DeFries, J. C., McClearn, G. E., & Rutter, M. (1997).
Behavioral genetics (3rd ed.). New york: W. H. Freeman.
Poelmans, G., Pauls, D. L., Buitelaar, J. K., & Franke, B. (2011). Integrated genome-wide association study findings: identification of a neurodevelopmental network for attention deficit hyperactivity disorder. American Journal of Psychiatry, online first, doi: 10.1176/appi.ajp.2010.10070948.
Polanczyk, G., Silva de Lima, M., Horta, B. L., Biederman, J., & Rohde,
L. A. (2007). The worldwide prevalence of ADHD: A systematic review and
metaregression analysis. American Journal of Psychiatry, 164, 942-948.
Porrino, L. J., Rapoport, J. L., Behar, D., Sceery, W., Ismond, D. R., &
Bunney, W. E., Jr. (1983). A naturalistic assessment of the motor activity of
hyperactive boys. Archives of General Psychiatry, 40, 681-687.
Quay, H. C. (1997). Inhibition and attention deficit hyperactivity disorder.
Journal of Abnormal Child Psychology, 25, 7-13.
Rabiner, D., Coie, J. D., and the Conduct Problems Prevention Research Group.
(2000). Early attention problems and children’s reading achievement:
A longitudinal investigation. Journal of the American Academy of Child
and Adolescent Psychiatry, 39, 859-867.
Rapoport, J. L., Buchsbaum, M. S., Zahn, T. P., Weingarten, H., Ludlow, C.,
& Mikkelsen, E. J. (1978). Destroamphetamine: Cognitive and behavioral
effects in normal prepubertal boys. Science, 199, 560-563.
Rapoport, J. L., Donnelly, M., Zametkin, A., & Carrougher, J. (1986). “Situational
hyperactivity” in a U.S. clinical setting. Journal of Child Psychology
and Psychiatry, 27, 639-646.
Rapport, M. D., Scanlan, S. W., & Denney, C. B. (21999). Attention-deficit/hyperactivity
disorder and scholastic achievement: A model of dual developmental pathways.
Journal of Child Psychology and Psychiatry, 40, 1169-1183.
Rapport, M. D., Tucker, S. B., DuPaul, G. J., Merlo, M., & Stoner, G. (1986).
Hyperactivity and frustration: The influence of control over and size of rewards
in delaying gratification. Journal of Abnormal Child Psychology, 14, 181-204.
Raskin, L. A. Shaywitz, S. E., Shaywitz, B. A., Anderson, G. M., & Cohen,
D. J. (1984). Neurochemical correlates of attention deficit disorder.
Pediatric Clinics of North America, 31, 387-396.
Rasmussen, P., & Gillberg, C. (2001). Natural outcome of ADHD with
developmental coordination disorder at age 22 years: A controlled, longitudinal,
community-based study. Journal of the American Academy of Child and Adolescent
Psychiatry, 39, 1424-1431.
Reebye, P. N. (1997). Diagnosis and treatment of ADHD in preschoolers.
Paper presented at the annual meeting of the American Academy of Child and Adolescent
Psychiatry, Toronto, October.
Richman, N., Stevenson, J., & Graham, P. (1982). Preschool to school: A
behavioural study. New York: Academic Press.
Roberts, M. A. (1990). A behavioral observation method for differentiating
hyperactive and aggressive boys. Journal of Abnormal Child Psychology, 18, 131-142.
Rodriquez, A., Olsen, J., Kotimaa, A. J., Kaakinen, M., Moilanen, I., Henriksen, T. B., Linnet, K. M., Miettunen, J., Obel, C., Taanila, A., Ebeling, H., & Jarvelin, M. R. (2009). Is prenatal alcohol exposure related to inattention and hyperactivity symptoms in children? Disentangling the effects of social adversity. Journal of Child Psychology and Psychiatry, 50, 1073-1083.
Rohde, L. A., Biederman, J., Busnello, E. A., Zimmermann, H., Schmitz, M.,
Martins, S., & Tramontina, S. (1999). ADHD in a school sample of Brazilian
adolescents: A study of prevalence, comorbid conditions, and impairments.
Journal of the American Academy of Child and Adolescent Psychiatry, 38, 716-722.
Roizen, N. J., Blondis, T. A., Irwin, M., & Stein, M. (1994). Adaptive
functioning in children with attention-deficit hyperactivity disorder.
Archives of Pediatric and Adolescent Medicine, 148, 1137-1142.
Roth, N., Beyreiss, J., Schlenzka, K., & Beyer, H. (1991). Coincidence
of attention deficit disorder and atopic disorders in children: Empirical findings
and hypothetical background. Journal of Abnormal Child Psychology, 19, 1-13.
Rothenberger, A. (1995). Electrical brain activity in children with hyperkinetic
syndrome: Evidence of a frontal cortical dysfunction. In J. A. Sergeant
(Ed.), Eunethydis: European approaches to hyperkinetic disorder (pp. 255-270).
Amsterdam: Author.
Routh, D. K., & Schroeder, C. S. (1976). Standardized playroom measures
as indices of hyperactivity. Journal of Abnormal Child Psychology, 4, 199-207.
Rubia, K., Overmeyer, S., Taylor, E., Brammer, M., Williams, S. C. R., Simmons,
A., & Bullmore, E. T. (1999). Hypofrontality in attention deficit
hyperactivity disorder during higher-order motor control: A study with functional
MRI. American Journal of Psychiatry, 156, 891-896.
Rucklidge, J. J., & Tannock, R. (2001). Psychiatric, psychosocial,
and cognitive functioning of female adolescents with ADHD. Journal of
the American Academy of Child and Adolescent Psychiatry, 40, 530-540.
Russo, M. F., & Beidel, D. C. (1994). Comorbidity of childhood anxiety
and externalizing disorders: Prevalence, associated characteristics, and validation
issues. Clinical Psychology Review, 14, 199-221.
Rutter, M. (1977). Brain damage syndromes in childhood: Concepts and findings.
Journal of Child Psychology and Psychiatry, 18, 1-21.
Rutter, M., Bolten, P., Harrington, R., LeCouteur, A., Macdonald, H., &
Simonoff, E. (1990). Genetic factors in child psychiatric disorders – I.
A review of research strategies. Journal of Child Psychology and Psychiatry,
31, 3-37.
Rutter, M., Macdonald, H., LeCouteur, A., Harrington, R., Bolton, P., &
Bailey, P. (1990). Genetic factors in child psychiatric disorders – II.
Empirical findings. Journal of Child Psychology and Psychiatry, 31, 39-83.
Sachs, G. S., Baldassano, C. F., Truman, C. J., & Guille, C. (2000).
Comorbidity of attention deficit hyperactivity disorder with early- and late-onset
bipolar disorder. American Journal of Psychiatry, 157, 466-468.
Sagvolden, T., Johansen, E. P., Ase, H., & Russell, V. A. (2005).
A dynamic developmental theory of attention-deficit hyperactivity disorder (ADHD)
predominantly hyperactive/impulsive and combined types. Behavioral and
Brain Sciences, 28, 397-468.
Samuel, V. J., George, P., Thornell, A., Curtis, S., Taylor, A., Brome, D.,
Mick, E., Faraone, S. V., & Biederman, J. (1999). A pilot controlled
family study of DSM-III-R and DSM-IV ADHD in African-American children.
Journal of the American Academy of Child and Adolescent Psychiatry, 38, 34-39.
Sanchez, R. P., Lorch, E. P., Milich, R., & Welsh, R. (1999). Comprehension
of televised stories in preschool children with ADHD. Journal of Clinical
Child Psychology, 28, 376-385.
Satterfield, J. H., Hoppe, C. M., & Schell, A. M. (1982). A prospective
study of delinquency in 110 adolescent boys with attention deficit disorder
and 88 normal adolescent boys. American Journal of Psychiatry, 139, 795-
798.
Schachar, R. J., & Logan, G. D. (1990). Impulsivity and inhibitory control
in normal development and childhood psychopathology. Developmental Psychology,
26, 710-720.
Schachar, R., Rutter, M., & Smith, A. (1981). The characteristics of situationally
and pervasively hyperactive children: Implications for syndrome definition.
Journal of Child Psychology and Psychiatry, 22, 375-392.
Schachar, R. J., Tannock, R., & Logan, G. (1993). Inhibitory control, impulsiveness,
and attention deficit hyperactivity disorder. Clinical Psychology Review, 13,
721-740.
Schachar, R., Taylor, E., Weiselberg, M., Thorley, G., & Rutter, M. (1987).
Changes in family function and relationships in children who respond to methylphenidate.
Journal of the American Academy of Child and Adolescent Psychiatry, 26, 728-732.
Schatz, D. B., & Rostain, A. L. (2006). ADHD with comorbid anxiety: a review of the current literature. Journal of Attention Disorders, 10, 141-149.
Scheres, A., Oosterlaan, J., & Sergeant, J. A. (2001). Response execution
and inhibition in children with AD/HD and other disruptive disorders: The role
of behavioural activation. Journal of Child Psychology and Psychiatry,
42, 347-357.
Schleifer, M., Weiss, G., Cohen, N. J., Elman, M., Cvejic, H., & Kruger,
E. (1975). Hyperactivity in preschoolers and the effect of methylphenidate.
American Journal of Orthopsychiatry, 45, 38-50.
Schothorst, P. F., & van Engeland, H. (1996). Long-term behavioral
sequelae of prematurity. Journal of the American Academy of Child and
Adolescent Psychiatry, 35, 175-183.
Schrag, P., & Divoky, D. (1975). The myth of the hyperactive child. New
York: Pantheon.
Schweitzer, J. B., Faber, T. L., Grafton, S. T., Tune, L. E., Hoffman, J. M.,
Kilts, C. D. (2000). Alterations in the functional anatomy of working memory
in adult attention deficit hyperactivity disorder. American Journal of
Psychiatry, 157, 278-280
Seidman, L. J., Benedict, K. B., Biederman, J., Bernstein, J. H., Seiverd,
K., Milberger, S., Norman, D., Mick, E., & Faraone, S. V. (1995).
Performance of children with ADHD on the Rey-Osterrieth Complex Figure:
A pilot neuropsychological study. Journal of Child Psychology and Psychiatry,
36, 1459-1473.
Seidman, L. J., Biederman, J., Faraone, S. V., Milberger, S., Norman, D., Seiverd,
K., Benedict, K., Guite, J., Mick, E., & Kiely, K. (1995). Effects
of family history and comorbidity on the neuropsychological performance of children
with ADHD: Preliminary findings. Journal of the American Academy of Child
and Adolescent Psychiatry, 34, 1015-1024.
Semrud-Clikeman, M., Biederman, J., Sprich-Buckminster, S., Lehman, B. K.,
Faraone, S. V., & Norman, D. (1992). Comorbidity between ADDH and
learning disability: A review and report in a clinically referred sample.
Journal of the American Academy of Child and Adolescent Psychiatry, 31, 439-448.
Sergeant, J. (1988). From DSM-III attentional deficit disorder to functional
defects. In L. Bloomingdale & J. Sergeant (Eds.), Attention deficit disorder:
Criteria, cognition, and intervention (pp. 183-198). New York: Pergamon.
Sergeant, J. A. (2005). Modeling attention-deficit/hyperactivity disorder: a critical appraisal of the cognitive-energetic model. Biological Psychiatry, 57, 1248-1255.
Sergeant, J., & van der Meere, J. P. (1994). Toward an empirical child
psychopathology. In D. K. Routh (Ed.), Disruptive behavior disorders in children
(pp. 59-86). New York: Plenum Press.
Shaywitz, S. E., Shaywitz, B. A., Cohen, D. J., & Young, J. G. (1983).
Monoaminergic mechanisms in hyperactivity. In M. Rutter (Ed.), Developmental
neuropsychiatry (pp. 330-347). New York: Guilford.
Shaywitz, S. E., Shaywitz, B. A., Jatlow, P. R., Sebrechts, M., Anderson, G.
M., & Cohen, D. J. (1986). Biological differentiation of attention
deficit disorder with and without hyperactivity. A preliminary report.
Annals of Neurology, 21, 363.
Shelton, T. L., Barkley, R. A., Crosswait, C., Moorehouse, M., Fletcher, K.,
Barrett, S., Jenkins, L., & Metevia, L. (1998). Psychiatric and psychological
morbidity as a function of adaptive disability in preschool children with high
levels of aggressive and hyperactive-impulsive-inattentive behavior. Journal
of Abnormal Child Psychology, 26, 475-494.
Shiels, K. & Hawk, L. W. Jr. (2010). Self-regulation in ADHD: the role of error processing. Clinical Psychology Review, 30, 951-961.
Silva, P. A., Hughes, P., Williams, S., & Faed, J. M. (1988). Blood lead,
intelligence, reading attainment, and behaviour in eleven year old children
in Dunedin, New Zealand. Journal of Child Psychology and Psychiatry, 29, 43-52.
Silverman, I. W., & Ragusa, D. M. (1992). Child and maternal correlates
of impulse control in 24-month old children. Genetic, Social, and General Psychology
Monographs, 116, 435-473.
Simmell, C., & Hinshaw, S. P. (1993, March). Moral reasoning and antisocial
behavior in boys with ADHD. Poster presented at the biennial meeting of the
Society for Research in Child Development, New Orleans.
Simpson, H. A., Jung, L., & Murphy, T. K. (2011). Update on attention-deficit/hyperactivity disorder and tic disorders: a review of the current literature. Current Psychiatry Reports, 13, 351-356.
Skirrow, C., Hosang, G. M., Farmer, A. E., & Asherson, P. (2012). An update on the debated association between ADHD and bipolar disorder across the lifespan. Journal of Affective Disorders, online first, doi:10.1016/j.jad.2012.04.003.
Slusarek, M., Velling, S., Bunk, D., & Eggers, C. (2001). Motivational
effects on inhibitory control in children with ADHD. Journal of the American
Academy of Child and Adolescent Psychiatry, 40, 355-363.
Smalley, S. L., McGough, J. J., Del’Homme, M., NewDelman, J., Gordon,
E., Kim, T., Liu, A., & McCracken, J. T. (2000). Familial clustering
of symptoms and disruptive behaviors in multiplex families with attention-deficit/hyperactivity
disorder. Journal of the American Academy of Child and Adolescent Psychiatry,
39, 1135-1143.
Smith, A. K., Mick, E., & Faraone, S. V. (2009). Advances in genetic studies of attention-deficit/hyperactivity disorder. Current Psychiatry Reports, 11, 143-148.
Sonuga-Barke, E. J., Lamparelli, M., Stevenson, J., Thompson, M., & Henry,
A. (1994). Behaviour problems and pre-school intellectual attainment: The associations
of hyperactivity and conduct problems. Journal of Child Psychology and Psychiatry,
35, 949-960.
Sonuga-Barke, E. J. S., Taylor, E., & Hepinstall, E. (1992).
Hyperactivity and delay aversion II. The effect of self versus externally
imposed stimulus presentation periods on memory. Journal of Child Psychology
and Psychiatry, 33, 399-409.
Solanto, M. V., Abikoff, H., Sonuga-Barke, E., Schachar, R., Logan, G. D.,
Wigal, T., Hechtman, L., Hinshaw, S., & Turkel, E. (2001). The ecological
validity of delay aversion and response inhibition as measures of impulsivity
in AD/HD: A supplement to the NIMH Multimodal Treatment Study of ADHD.
Journal of Abnormal Child Psychology, 29, 215-228.
Sonuhara, G. A., Barr, C., Schachar, R. J., Tannock, R., Roberts, W., Malone,
M. A., Jain, U. R., & Kennedy, J. L. (1997). Association study of
the dopamine D4 receptor gene in children and adolescents with ADHD. Paper
presented at the annual meeting of the American Academy of Child and Adolescent
Psychiatry, Toronto, October.
Southam-Gerow, M. A., & Kendall, P. C. (2002). Emotion regulation
and understanding: Implications for child psychopathology and therapy.
Clinical Psychology Review, 22, 189-222.
Spencer, T. J., Biederman, J., Faraone, S., Mick, E., Coffey, B., Geller, D.,
Kagan, J., Bearman, S. K., & Wilens, T. (2001). Impact of tic disorders
on ADHD outcome across the life cycle: findings from a large group of adults
with and without ADHD. American Journal of Psychiatry, 158, 6110617.
Spencer, T. J., Biederman, J., Harding, M., O’Donnell, D., Faraone, S.
V., & Wilens, T. E. (1996). Growth deficits in ADHD children revisited:
Evidence for disorder-associated growth delays? Journal of the American
Academy of Child and Adolescent Psychiatry, 35, 1460-1469.
Spencer, T., Wilens, T., Biederman, J., Wozniak, J., & Harding-Crawford,
M. (2000). Attention-deficit/hyperactivity disorder with mood disorders.
In T. E. Brown (Ed.), Attention deficit disorders and comorbidities in children,
adolescents, and adults (pp. 79-124). Washington, DC: American Psychiatric
Press.
Sprich, S., Biederman, J., Crawford, M. H., Mundy, E., & Faraone, S. V.
(2000). Adoptive and biological families of children and adolescents with
ADHD. Journal of the American Academy of Child and Adolescent Psychiatry,
39, 1432-1437.
Stavrinos, D., Biasini, F. J., Fine, P. R., Hodgens, J. B., Khatri, S., Mrug, S., Schwebel, D. C. (2011). Mediating factors associated with pedestrian injury in children with attention-deficit/hyperactivity disorder. Pediatrics, online first, doi:10.1542/peds.2010-3829.
Stein, M. A. (1999). Unravelling sleep problems in treated and untreated
children with ADHD. Journal of Child and Adolescent Psychopharmacology,
9, 157-168.
Stein, M. A., Szumowski, E., Blondis, T. A., & Roizen, N. J. (1995). Adaptive
skills dysfunction in ADD and ADHD children. Journal of Child Psychology and
Psychiatry, 36, 663-670.
Stein, M. A., Weiss, R. E., & Refetoff, S. (1995). Neurocognitive
characteristics of individuals with resistance to thyroid hormone: Comparisons
with individuals with attention-deficit hyperactivity disorder. Journal
of Developmental and Behavioral Pediatrics, 16, 406-411.
Stevenson, J., Pennington, B. F., Gilger, J. W., DeFries, J. C., & Gilies,
J. J. (1993). Hyperactivity and spelling disability: Testing for shared genetic
aetiology. Journal of Child Psychology and Psychiatry, 34, 1137-1152.
Stewart, M. A. (1970). Hyperactive children. Scientific American, 222, 94-98.
Stewart, M. A., Pitts, F. N., Craig, A. G., & Dieruf, W. (1966). The hyperactive
child syndrome. American Journal of Orthopsychiatry, 36, 861-867.
Stewart, M. A., Thach, B. T., & Friedin, M. R. (1970). Accidental poisoning
and the hyperactive child syndrome. Disease of the Nervous System, 31, 403-407.
Still, G. F. (1902). Some abnormal psychical conditions in children. Lancet,
1, 1008-1012, 1077-1082, 1163-1168.
Strauss, A. A., & Kephardt, N. C. (1955). Psychopathology and education
of the brain-injured child: Vol. 2. Progress in theory and clinic. New York:
Grune & Stratton.
Strauss, A. A., & Lehtinen, L. E. (1947). Psychopathology and education
of the brain-injured child. New York: Grune & Stratton.
Strauss, M. E., Thompson, P., Adams, N. L., Redline, S., & Burant, C. (2000).
Evaluation of a model of attention with confirmatory factor analysis.
Neuropsychology, 14, 201-208.
Stryker, S. (1925). Encephalitis lethargica – The behavior residuals. Training
School Bulletin, 22, 152-157.
Stuss, D. T., & Benson, D. F. (1986). The frontal lobes. New York: Raven
Press.
Swaab-Barneveld, H., DeSonneville, L., Cohen-Kettenis, P., Gielen, A., Buitelaar,
J., & van Engeland, H. (2000). Visual sustained attention in a child
psychiatric population. Journal of the American Academy of Child and Adolescent
Psychiatry,39, 651-659.
Sykes, D. H., Hoy, E. A., Bill, J. M., McClure, B. G., Halliday, H. L., &
Reid, M. M. (1997). Behavioural adjustment in school of very low birthweight
children. Journal of Child Psychology and Psychiatry, 38, 315-325.
Szatmari, P. (1992). The epidemiology of attention-deficit hyperactivity disorders.
In G. Weiss (Ed.), Child and adolescent psychiatric clinics of North America:
Attention-deficit hyperactivity disorder (pp. 361-372). Philadelphia:
Saunders.
Szatmari, P., Offord, D. R., & Boyle, M. H. (1989). Correlates, associated
impairments, and patterns of service utilization of children with attention
deficit disorders: Findings from the Ontario Child Health Study. Journal of
Child Psychology and Psychiatry, 30, 205-217.
Szatmari, P., Saigal, S., Rosenbaum, P. & Campbell, D. (1993). Psychopathology
and adaptive functioning among extremely low birthweight children at eight years
of age. Development and Psychopathology, 5, 345-357.
Tallmadge, J., & Barkley, R. A. (1983). The interactions of hyperactive
and normal boys with their mothers and fathers. Journal of Abnormal Child Psychology,
11, 565-579.
Tannock, R. (2009). Attention-deficit/hyperactivity disorder with anxiety disorders. In T. E. Brown (Ed.), ADHD Comorbidities (pp. 131-156). Washington, DC: American Psychiatric Press.
Tannock, R., & Brown, T. E. (2009). Attention-deficit disorders with learning disorders in children and adolescents. In T. E. Brown (Ed.), ADHD comorbidities (pp. 189-232). Washington, DC: American Psychiatric Press.
Tannock, R., Martinussen, R., & Frijters, J. (2000). Naming speed
performance and stimulant effects indicate effortful, semantic processing deficits
in attention-deficit/hyperactivity disorder. Journal of Abnormal Child
Psychology, 28, 237-252.
Tarver-Behring, S., Barkley, R. A., & Karlsson, J. (1985). The mother-child
interactions of hyperactive boys and their normal siblings. American Journal
of Orthopsychiatry, 55, 202-209.
Taylor, E. (1999). Developmental neuropsychology of attention deficit
and impulsiveness. Development and Psychopathology, 11, 607-628.
Taylor, E., Sandberg, S., Thorley, G., & Giles, S. (1991). The epidemiology
of childhood hyperactivity. Oxford, UK: Oxford University Press.
Theule, J., Wiener, J., Rogers, M. A., & Marton, I. (2011). Predicting parenting stress in families of children with ADHD: parent and contextual factors. Journal of Child and Family Studies, 20, 640-647.
Tillman, C. M., Bohlin, G., Sorenson, L., & Lundercold, A. J. (2009). Intellectual deficits in children with ADHD beyond central executive and non-executive functions. Archives of Clinical Neuropsychology, 24, 269-282.
Torgesen, J. K. (1994). Issues in the assessment of of executive function:
An information-processing perspective. In G. R. Lyon (Ed.), Frames of reference
for the assessment of learning disabilities: New views on measurement issues
(pp. 143-162). Baltimore: Brookes.
Tripp, G., & Alsop, B. (1999). Sensitivity to reward frequency in
boys with attention deficit hyperactivity disorder. Journal of Clinical
Child Psychology, 28, 366-375.
Tripp, G., & Alsop, B. (2001). Sensitivity to reward delay in children
with attention deficit hyperactivity disorder (ADHD). Journal of Child
Psychology and Psychiatry, 42, 691-698.
Trommer, B. L., Hoeppner, J. B., Rosenberg, R. S., Armstrong, K. J., &
Rothstein, J. A. (1988). Sleep disturbances in children with attention deficit
disorder. Annals of Neurology, 24, 325.
Ullman, D. G., Barkley, R. A., & Brown, H. W. (1978). The behavioral
symptoms of hyperkinetic children who successfully responded to stimulant drug
treatment. American Journal of Orthopsychiatry, 48, 425-437.
Valera, E. M., Faraone, S. V., Murray, K. E., & Seidman, L. J. (2007). Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry, 61, 1361-1369.
Valo, S., & Tannock, R. (2010). Diagnostic instability of DSM-IV ADHD subtypes: Effects of informant, instrumentation, and methods for combining symptom reports. Journal of Clinical Child and Adolescent Psychology, 39, 749-760.
van den Oord, E. J. C. G., Boomsma, D. I., & Verhulst, F. C. (1994). A
study of problem behaviors in 10- to 15-year-old biologically related and unrelated
international adoptees. Behavior Genetics, 24, 193-205.
Vater, C. H., DiSalvo,, Ehrlich, A., Parker, H., O’Connor, H., Faraone, S.V., & Biederman, J., . (2024). ADHD in adults: Does age at diagnosis matter? Journal of Attention Disorders, 28(5): 614-624. https://doi.org/10.1177/10870547231218450
Velez, C. N., Johnson, J., & Cohen, P. (1989). A longitudinal analysis
of selected risk factors for childhood psychopathology. Journal of the American
Academy of Child and Adolescent Psychiatry, 28, 861-864.
Velting, O. N., & Whitehurst, G. J. (197). Inattention-hyperactivity
and reading achievement in children from low-income families: A longitudinal
model. Journal of Abnormal Child Psychology, 25, 321-331.
Voelker, S. L., Carter, R. A., Sprague, D. J., Gdowski, C. L., & Lachar,
D. (1989). Developmental trends in memory and metamemory in children with attention
deficit disorder. Journal of Pediatric Psychology, 14, 75-88.
Vohs, K. D., & Baumeister, R. F. (Eds.) (2011). Handbook of self-regulation: Research, theory, and applications (2nd ed). New York: Guilford Press.
Vygotsky, L. S. (1978). Mind in society. Cambridge, MA: Harvard University
Press.
Vygotsky, L. S. (1987). Thinking and speech. In The collected works of L. S.
Vygotsky: Vol. 1. Problems in general psychology (N. Minick, Trans.). New York:
Plenum Press.
Waschbusch, D. A. (2002). A meta-analytic examination of comorbid
hyperactive-impulsive-attention problems and conduct problems. Psychological
Bulletin, 128, 118-150.
Wakefield, J. C. (1999). Evolutionary versus prototype analyses of the
concept of disorder. Journal of Abnormal Psychology, 108, 374-399.
Wallander, J. L., Schroeder, S. R., Michelli, J. A., & Gualtieri, C. T.
(1987). Classroom social interactions of attention deficit disorder with hyperactivity
children as a function of stimulant medication. Journal of Pediatric Psychology,
12, 61-76.
Weiss, G., & Hechtman, L. (1993). Hyperactive children grown up (2nd ed.).
New York: Guilford Press.
Welner, Z., Welner, A., Stewart, M., Palkes, H., & Wish, E. (1977). A controlled
study of siblings of hyperactive children. Journal of Nervous and Mental Disease,
165, 110-117.
Welsh, M. C., & Pennington, B. F. (1988). Assessing frontal lobe functioning
in children: Views from developmental psychology. Developmental Neuropsychology,
4, 199-230.
Werry, J. S., Elkind, G. S., & Reeves, J. S. (1987). Attention deficit,
conduct, oppositional, and anxiety disorders in children: III. Laboratory differences.
Journal of Abnormal Child Psychology, 15, 409-428.
Werry, J. S., & Quay, H. C. (1971). The prevalence of behavior symptoms
in younger elementary school children. American Journal of Orthopsychiatry,
41, 136-143.
Whalen, C. K., & Henker, B. (1992). The social profile of attention-deficit
hyperactivity disorder: Five fundamental facets. In G. Weiss (Ed.), Child and
adolescent psychiatric clinics of North America: Attention-deficit hyperactivity
disorder (pp. 395-410). Philadelphia: Saunders.
Whalen, C. K., Henker, B., Collins, B. E., McAuliffe, S., & Vaux, A. (1979).
Peer interaction in structured communication task: Comparisons of normal and
hyperactive boys and of methylphenidate (Ritalin) and placebo effects. Child
Development, 50, 388-401.
Whalen, C. K., Henker, B., & Dotemoto, S. (1980). Methylphenidate and hyperactivity:
Effects on teacher behaviors. Science, 208, 1280-1282.
Whalen, C. K., Henker, B., Swanson, J. M., Granger, D., Kliewer, W., &
Spencer, J. (1987). Natural social behaviors in hyperactive children: Dose effects
of methylphenidate. Journal of Consulting and Clinical Psychology, 55, 187-193.
Whittaker, A. H., Van Rossem, R., Feldman, J. F., Schonfeld, I. S., Pinto-Martin,
J. A., Torre, C., Shaffer, D., & Paneth, N. (1997). Psychiatric outcomes
in low-birth-weight children at age 6 years: Relation to neonatal cranial ultrasound
abnormalities. Archives of General Psychiatry, 54, 847-856.
Wilens, T. E., Biederman, J., & Spencer, T. (1994). Clonidine for
sleep disturbances associated with attention-deficit hyperactivity disorder.
Journal of the American Academy of Child and Adolescent Psychiatry, 33, 424-426.
Willcutt, E. G., Pennington, B. F., Boada, R., Ogline, J. S., Tunick, R. A.,
Chhabildas, N. A., & Olson, R. K. (2001). A comparison of the cognitive
deficits in reading disability and attention-deficit/hyperactivity disorder.
Journal of Abnormal Psychology, 110, 157-172.
Willcutt, E. G., Pennington, B. F., Chhabildas, N. A., Friedman, M. C., &
Alexander, J. (1999). Psychiatric comorbidity associated with DSM-IV ADHD
in a nonreferred sample of twins. Journal of the American Academy of Child
and Adolescent Psychiatry,38, 1355-1362.
Wilcutt, E. G., Doyle, A. E., Nigg, J. T., Faraone, S. V., & Pennington, B. F. (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57, 1336-1346.
Willerman, L. (1973). Activity level and hyperactivity in twins. Child Development,
44, 288-293.
Willis, T. J., & Lovaas, I. (1977). A behavioral approach to treating hyperactive
children: The parent’s role. In J. B. Millichap (Ed.), Learning disabilities
and related disorders (pp. 119-140). Chicago: Yearbook Medical Publications.
Winsler, A. (1998). Parent-child interaction and private speech in boys
with ADHD. Applied Developmental Science, 2, 17-39.
Winsler, A., Diaz, R. M., Atencio, D. J., McCarthy, E. M., & Chabay, L.
A. (2000). Verbal self-regulation over time in preschool children at risk
for attention and behavior problems. Journal of Child Psychology and Psychiatry,
41, 875-886.
Wood, F. B., & Felton, R. H. (1994). Separate linguistic and attentional
factors in the development of reading. Topics in language disorders, 14, 52-57.
Woodward, L. J., Fergusson, D. M., & Horwood, L. J. (2000). Driving
outcomes of young people with attentional difficulties in adolescence.
Journal of the American Academy of Child and Adolescent Psychiatry, 39, 627-634.
World Health Organization. (1993). The ICD-10 classification of mental and
behavioral disorders: Diagnostic criteria for research. Geneva, Switzerland:
Author.
Wozniak, J., Biederman, J., Kiely, K., Ablon, S., Faraone, S. V., Mundy, E.,
& Mennin, D. (1995). Mania-like symptoms suggestive of childhood-onset
bipolar disorder in clinically referred children. Journal of the American
Academy of Child and Adolescent Psychiatry, 34, 867-876.
Wu, J., Xiao, H., Sun, H., Zou, L., & Zhu, L. (2012). Role of dopamine receptors in ADHD: a systematic meta-analysis. Molecular Neurobiology, 45, 605-620.
Youngstrom, E. A., Arnold, L. E., & Frazier, T. W. (2010). Bipolar and ADHD comorbidity: both artifact and outgrowth of shared mechanisms. Clinical Psychology: Science and Practice, 17, 350-359.
Zagar, R., & Bowers, N. D. (1983). The effect of time of day on problem-solving
and classroom behavior. Psychology in the Schools, 20, 337-345.
Zametkin, A. J., Liebenauer, L. L., Fitzgerald, G. A., King, A. C., Minkunas,
D. V., Herscovitch, P., Yamada, E. M., & Cohen, R. M. (1993). Brain metabolism
in teenagers with attention-deficit hyperactivity disorder. Archives of General
Psychiatry, 50, 333-340.
Zametkin, A. J., Nordahl, T. E., Gross, M., King, A. C., Semple, W. E., Rumsey,
J., Hamburger, S., & Cohen, R. M. (1990). Cerebral glucose metabolism in
adults with hyperactivity of childhood onset. New England Journal of Medicine,
323, 1361-1366.
Zametkin, A. J., & Rapoport, J. L. (1986). The pathophysiology of
attention deficit disorder with hyperactivity: A review. In B. B. Lahey
& A. E. Kazdin (Eds.), Advances in clinical child psychology (Vol. 9, pp.
177-216). New York: Plenum.
Zentall, S. S. (1985). A context for hyperactivity. In K. Gadow & I. Bialer
(Eds.), Advances in learning and behavioral disabilities (Vol. 4, pp. 273-343).
Greenwich, CT: JAI Press.
Zentall, S. S. (1988). Production deficiencies in elicited language but not
in the spontaneous verbalizations of hyperactive children. Journal of Abnormal
Child Psychology, 16, 657-673.
Zentall, S. S., & Smith, Y. S. (1993). Mathematical performance and behavior
of children with hyperactivity with and without coexisting aggression. Behavior
Research and Therapy, 31, 701-710.
REFERENCES ON HEALTH OUTCOMES
Barbaresi, W. J., Colligan, R. C., Weaver, A. L., Voigt, R. G., Killian, J. M., Katusic, S. K. (2013). Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: A prospective study. Pediatrics, 131, 637-644.
Barkley, R. A. (2015c). Health problems and related impairments in children and adults with ADHD. In R. A. Barkley (ed.) Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th Ed)(pp. 267-313). New York, NY: Guilford Press.
Barkley, R. A. (2000). Adverse health outcomes of ADHD by adulthood. The ADHD Report, 28(4), 1-6.
Barkley, R. A. & Cox, D. J. (2007). A review of driving risks and impairments associated with Attention-Deficit/Hyperactivity Disorder and the effects of stimulant medication on driving performance. Journal of Safety Research, 38, 113-128.
Barkley, R. A. & Fischer, M. (2019). Hyperactive child syndrome and estimated life expectancy at young adult follow-up: The role of ADHD persistence and other potential predictors. Journal of Attention Disorders, 23, 907-923.
Barkley, R. A., Murphy, K. R., & Fischer, M. (2008). ADHD in adults: What the science says. New York: Guilford Press.
Bogg, T. & Roberts, B. W. (2004). Conscientiousness and health-related behavior: A meta-analysis of the leading behavioral contributors to mortality. Psychological Bulletin, 130, 887-919.
Boland, H., DiSalvo, M. Fried, R., Woodworth, K. Y., Wilens, T. Faraone, S. V., & Biederman, J. (2020). A literature review and meta-analysis on the effects of AHD medications on functional outcomes. Journal of Psychiatric Research, 123, 21-30.
Buitelaar, J. N. J., Posthumus, J. A., & Buitelaar, J. K. (2015). ADHD in childhood and/or adulthood as a risk factor for domestic violence or intimate partner violence: a systematic review. Journal of Attention Disorders. ePub ahead of print, doi: 10.1177/1087054715587099.
Chen, M. H et al. (2018). Risk of Type 2 diabetes in adolescents and young adults with attention-deficit/hyperactivity disorder: A nationwide longitudinal study. Journal of Clinical Psychiatry, 79(3), 17m11607.
Chen, J. J. et al. (2013). Association of attention-deficit/hyperactivity disorder with diabetes: a population-based study. Pediatric Research, 73, 492-496. Also Chen, M. H et al. (2018). Risk of Type
Chen, V.C.H., et al. (2019). Attention-deficit/hyperactivity disorder and mortality risk in Taiwan. JAMA Network. Epub ahead of print.
Chesney, E. et al. (2014). Risks for all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry, 13, 153-160.
Dalsgaard, S., Ostergaard, S. D., Leckman, J. F., Mortensen, P. B., & Pedersen, M. G. (2015). Mortality in children, adolescents and adults with attention deficit hyperactivity disorder: a nationwide cohort study. Lancet, 385, 2190-2196.
Demontis, D. et al. (2018). Discovery of the first genome wide association significant risk loci for attention-deficit/hyperactivity disorder. Nature Genetics. Epub ahead of print. doi.org/10.1038/s41588-018-0269-7
Fekri, N. et al. (2019). Association of body mass index with life expectancy with and without cardiovascular disease. International Journal of Obesity. Epub ahead of print.
Friedman, H. S., Tucker, J. S., Schwartz, J. E., Tomlinson-Keasey, C., Martin, L. R., Wingard, D. L., & Criqui, M. H. (1995). Psychosocial and behavioral predictors of longevity: The aging and death of the “Termites.” American Psychologist, 50, 69–78.
Goodwin, R. D. et al. (2009). Do mental health problems in childhood predict chronic physical conditions among males in early adulthood? Evidence from a community based prospective study. Psychological Medicine, 39(2), 301-311.
Hampson, S. E. (2008). Mechanisms by which childhood personality traits influence adult well-being. Current Directions in Psychological Science, 17, 264-268.
Jokela, M., Ferrie, J. E., & Kivimaki, M. (2008). Childhood problem behaviors and death by midlife: The British National Child Development Study. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 19-24.
Joshi, P. K., Pirastu, N., Kentistou, K. A., Fischer, K., Hofer, E., Schraut, K. E. et al. (2017). Genome-wide meta-analysis associates HLA-DQA1/DRB1 and LPA and lifestyle factors with human longevity. Nature Communications. Epub ahead of print: doi: 10.1038/s41467-017-00934-5.
Klein, R. G., Mannuzza, S., Olazagasti, M. A. R., Roizen, E., Hutchinson, J. A., Lashua, E. C., & Castellanos, X. (2012). Clinical and functional outcome of childhood attention-deficit/hyperactivity disorder 33 years later. Archives of General Psychiatry, 69, 1295-1303.
Li et al. (2018). Circulation, 138, 345-355.
London, A. S., & Landes, S. D. (2016). Attention deficit hyperactivity disorder and adult mortality. Preventive Medicine, 90, 8-10.
Makela, P. (1998). Alcohol related mortality by age and sex and its impact on life expectancy: Estimates based on the Finnish death register. European Journal of Public health, 8(1), 43-51.
Mohr-Jensen, C., & Steinhausen, H. C. (2016). A meta-analysis and systematic review of the risks associated with childhood attention-deficit hyperactivity disorder on long-term outcome of arrests, convictions, and incarcerations. Clinical Psychology Review, 48, 32-42.
Nigg, J. T. (2013). Attention-deficit/hyperactivity disorder and adverse health outcomes. Clinical Psychology Review, 33, 215-228.
Saylor, K. E. & Amann, B. H. (2016). Impulsive aggression as a comorbidity of attention-deficit/hyperactivity disorder in children and adolescents. Journal of Child and Adolescent Psychopharmacology, 26, 19-25.
Sun, S. et al. (2019). Association of psychiatric comorbidity with the risk of premature death among children and adults with attention-deficit hyperactivity disorder. JAMA Psychiatry. Epub ahead of print.
Virtanen, M., Lallukka, T., Alexanderson, K., Ervasti, J., Josefsson, P., Kivimaki, M., & Mittendorfer-Rutz, E. (2018). Work disability and mortality in early onset neuropsychiatric and behavioral disorders in Sweden. European Journal of Public Health, 28, Supplement 4, p. 32.
REFERENCES on CDS
Abikoff, H. (1985). Efficacy of cognitive training interventions in hyperactive children: A critical review. Clinical Psychology Review, 5, 479-512.
Achenbach, T. M. (2001). Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington, VT: Thomas Achenbach.
Achenbach, T. M., McConaughy, S. H., & Howell, C. T. (1987). Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychological Bulletin, 101, 213-232.
Adams, Z. W., Milich, R., & Fillmore, M. T. (2010). A case for the return of attention-deficit disorder in DSM-5-TR. The ADHD Report, 18(3), 1-6.
American Educational Research Association, American Psychological Association, and American Council on Measurement in Education. (2013). Standards for Educational and Psychological Testing. Washington, DC: AERA Publications
American Psychiatric Association. (1980). Diagnostic and statistical manual of mental disorders (3rd ed.). Washington, DC: Author.
American Psychiatric Association. (1987). Diagnostic and statistical manual of mental disorders (3rd ed.). Washington, DC: Author.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author.
American Psychiatric Association. (2022). Diagnostic and statistical manual of mental disorders (5th ed., test rev.). Washington, DC: Author.
Angold, A., Costello, E. J., & Erkanli, A. (1999). Comorbidity. Journal of Child Psychology and Psychiatry, 40, 57-88.
Antshel, K. M. & Remer, R. (2003). Social skills training in children with attention deficit hyperactivity disorder: a randomized-controlled clinical trial. Journal of Clinical Child and Adolescent Psychology, 32, 153-165.
Barkley, R. A. (1997). ADHD and the nature of self-control. New York: Guilford.
Barkley R. A. (2006). Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (3rd ed.). New York: Guilford Press.
Barkley, R. A. (2011a). The Barkley Adult ADHD Rating Scale - IV. New York: Guilford Press.
Barkley, R. A. (2011b). The Barkley Functional Impairment Scale. New York: Guilford Press.
Barkley, R. A. (2011c). The Barkley Deficits in Executive Functioning Scale. New York: Guilford.
Barkley, R. A. (2012a). Distinguishing sluggish cognitive tempo from attention deficit hyperactivity disorder in adults. Journal of Abnormal Psychology, 121, 978-990.
Barkley, R. A. (2012b). Executive Functions: What they are, how they work, and why they evolved. New York: Guilford Press.
Barkley, R. A. (2012c). The Barkley Functional Impairment Scale – Children and Adolescents. New York: Guilford Press.
Barkley, R. A. (2012d). The Barkley Deficits in Executive Functioning Scale – Children and Adolescents. New York: Guilford Press.
Barkley, R. A. (2013). Distinguishing sluggish cognitive tempo from ADHD in children and adolescents: executive functioning, impairment, and comorbidity. Journal of Clinical Child and Adolescent Psychology, 42, 161-173.
Barkley, R. A. (2014). Sluggish cognitive tempo (concentration deficit disorder?): current status, future directions, and a plea to change the name. Journal of Abnormal Child Psychology, 42, 117-125.
Barkley, R. A. (2015a). Concentration Deficit Disorder (Sluggish Cognitive Tempo). In R. A. Barkley (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th ed.) (pp. 435-454). New York: Guilford Press.
Barkley, R. A. (2015b). Executive functioning and self-regulation viewed as an extended phenotype: Implications of the theory for ADHD and its treatment. In R. A. Barkley (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th edition)(pp. 405-434). New York: Guilford Press.
Barkley, R. A. (2015c). Health problems and related impairments in children and adults. In R. A. Barkley (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th edition)(pp. 267-313). New York: Guilford Press
Barkley, R. A., Willcutt, E., & Jacobson, L. A. (2022). What is the cognitive deficit in sluggish cognitive tempo? A review of neuropsychological research. The ADHD Report, 30(4), 1-11.
Barkley, R. A., DuPaul, G. J., & McMurray, M.B. (1991). Attention deficit disorder with and without hyperactivity: Clinical response to three doses of methylphenidate. Pediatrics, 87, 519-531.
Barkley, R. A. & Fischer, M. (2011). Predicting impairment in major life activities and occupational functioning in hyperactive children as adults: Self-reported executive function (EF) deficits vs. EF tests. Developmental Neuropsychology, 36(2), 137-161.
Barkley, R. A., & Murphy, K. R. (2010). Impairment in occupational functioning and adult ADHD: The predictive utility of executive function (EF) ratings vs. EF tests. Archives of Clinical Neuropsychology, 25, 157-173.
Barkley, R. A., Murphy, K. R., & Fischer, M. (2008). ADHD in adults: What the science says. New York: Guilford Press.
Bauermeister, J. J., Barkley, R. A., Bauermeister, J. A., Martinez, J. V., & McBurnett, K. (2012). Validity of the sluggish cognitive tempo, inattention, and hyperactivity symptom dimensions: neuropsychological and psychosocial correlates. Journal of Abnormal Child Psychology, 40, 683-697.
Becker, S. P., Burns, G. L., Garner, A. A., Jarrett, M. A., Luebbe, A. M., Epstein, J. N., & Willcutt, E. G. (2017). Sluggish cognitive tempo in adults: Psychometric validation of the Adult Concentration Inventory. Psychological Assessment. Epub ahead of print.
Becker, S. P., Burns, G. L., Schmitt, A. P., Epstein, J. N., & Tamm, L. (2017). Toward establishing a standard symptom set for assessing Sluggish Cognitive Tempo in children: Evidence from teacher ratings in a community sample. Assessment. Epub ahead of print.
Becker, S. P., Garner, A. A., & Byars, K. C. (2016). Sluggish cognitive tempo in children referred to a pediatric Sleep Disorders Center: Examining possible overlap with sleep problems and associations with impairment. Journal of Psychiatric Research, 77, 116-124.
Becker, S. P., & Langberg, J. M. (2012). Sluggish cognitive tempo among young adolescents with ADHD: Relations to mental health, academic, and social functioning. Journal of Attention Disorders. Epub ahead of print.
Becker, S. P., & Langberg, J. M. (2013). Attention-deficit/hyperactivity disorder and sluggish cognitive tempo dimensions in relation to executive functioning in adolescents with ADHD. Child Psychiatry and Human Development, 45, 1-11.
Becker, S. P., Langberg, J. M., Luebbe, A. M., Dvorsky, M. R., & Flannery, A. J. (in press). Sluggish cognitive tempo is associated with academic functioning and internalizing symptoms in college students with and without attention-deficit/hyperactivity disorder. Journal of Clinical Psychology.
Becker, S. P. Leopold, D. R., Burns, G. L., Jarrett, M. A., Langberg, J. M., Marshall, S. A., McBurnett, K., Waschbusch, D. A., & Willcutt, E. G. (2016). Internal, external, and diagnostic validity of sluggish cognitive tempo: A meta-analysis and critical review. Journal of the American Academy of Child and Adolescent Psychiatry, 55, 163-178.
Becker, S. P., Luebbe, A. M., Fite, P. J., Stoppelbein, L., & Greening, L. (2013). Sluggish cognitive tempo in psychiatrically hospitalized children: factor structure and relations to internalizing symptoms, social problems, and observed behavioral dysregulation. Journal of Abnormal Child Psychology. Epub ahead of print.
Becker, S. P., Luebbe, A. M., & Langberg, J. M. (2014). Attention-deficit/hyperactivity disorder dimensions and sluggish cognitive tempo symptoms in relation to college students sleep functioning. Child Psychiatry and Human Development, 45, 675-685.
Becker, S. P., Willcutt, E. G., Leopold, D. R., Fredrick, J. W., Smith, Z. R., Jacoson, L. A., Burns, G. L., Mayes, S. D., Waschbusch, D. A., Froehlich, T. E., McBurnett, K., Servera, M., & Barkley, R. A. (2022). Report of a work group on sluggish cognitive tempo: Key research directions and a consensus change in terminology to Cognitive Disengagement Hypoactivity Syndrome (CDHS). Journal of the American Academy of Child and Adolescent Psychiatry. Epub ahead of print.
Belmar, M., Servera, M., Becker, S. P., & Burns, G. L. (2015). Validity of sluggish cognitive tempo in South America: An initial examination using mother and teacher ratings of Chilean children. Journal of Attention Disorders, 21, 667-672.
Biederman, J., Petty, C. R., Fried, R., Black, S., Faneuil, A., Doyle, A. E., Seidman, L. J., & Faraone, S. V. (2008). Discordance between psychometric testing and questionnaire-based definitions of executive function deficits in individuals with ADHD. Journal of Attention Disorders, 12, 92-102.
Boyle, C. A., Decoufle, P., & Yeargin-Allsop, M. (1994). Prevalence and health impact of developmental disabilities in US children. Pediatrics, 93, 399-403.
Brown, T. E. (2009) (Editor). ADHD comorbidities: Handbook for ADHD complications in children and adults. Arlington, VA: American Psychiatric Publishing, Inc.
Bryant, F. B. (2000). Assessing the validity of measurement. In. L. G. Grim and P. R. Yarnold (Eds.), Reading and understanding more multivariate statistics (pp. 99-146). Washington, DC: American Psychological Association.
Burns, C. L., Servera, M., Bernad, M. M., Carrillo, J. M., & Cardo, E. (2013). Distinctions between sluggish cognitive tempo, ADHD-IN, and depression symptom dimensions in Spanish first-grade children. Journal of Clinical Child and Adolescent Psychology, 42, 796-808.
Camprodon-Rosanas, E., Batlle, S., Estrada-Prat, X., Acena-Diaz, M., Petrizan-Aleman, A., Pujals, E., Martin-Lopez, L. M., Perez-Sola, V., & Ribas-Fito, N. (2016). Sluggish cognitive tempo in child and adolescent clinical outpatient setting. Journal of Psychiatric Practice, 22, 355-362.
Camprodon-Rosanas, E., Ribas-Fito, N., Batlle-Vila, S., Persavento, C., Alvarexz-Pedrerol, M., Sunyer, J., & Forns, J. (2016). Sluggish cognitive tempo: Sociodemographic, behavioral, and clinical characteristics in a population of Catalan school children. Journal of Attention Disorders, 21, 632-641.
Camprodon-Rosanas, E., Ribas-Fito, N., Batlle, S., Persavento, C., Alvarexz-Pedrerol, M., Sunyer, J., & Forns, J. (2017). Association between sluggish cognitive tempo symptoms and attentional network and working memory in primary schoolchildren. Journal of Attention Disorders. Epub ahead of print
Capdevila-Brophy, C., Artigas-Pallares, J., Nacarro-Pastor, J. B., Garcia-Nonell, K., Rigau-Ratera, E., & Obiols, J. E. (2012). ADHD predominantly inattentive subtype with high sluggish cognitive tempo: a new clinical entity? Journal of Attention Disorders, 18, 607-616.
Carlson, C. L. (1986). Attention deficit disorder with and without hyperactivity: A review of preliminary experimental evidence. In B. B. Lahey & A. E. Kazdin (Eds.), Advances in Clinical Child Psychology (Vol. 9, pp. 153-175). New York: Plenum.
Carlson, C. L., Lahey, B. B., & Neeper, R. (1986). Direct assessment of the cognitive correlates of attention deficit disorders with and without hyperactivity. Journal of Behavioral Assessment and Psychopathology, 8, 69–86.
Carlson, C. L., & Mann, M. (2002). Sluggish cognitive tempo predicts a different pattern of impairment in the attention deficit hyperactivity disorder, Predominantly Inattentive Type. Journal of Clinical Child and Adolescent Psychology, 31, 123-129.
Christoff, K., Irving, Z. C., Fox, K. C. R., Spreng, N., & Andrews-Hanna, A. (2016). Mind-wandering as spontaneous thought: a dynamic framework. Nature Reviews: Neuroscience, 17, 718-731.
Combs, M. A., Canu, W. H., Broman, J. J., & Nieman, D. C. (2013). Impact of sluggish cognitive tempo and attention-deficit/hyperactivity disorder symptoms on adults’ quality of life. Applied Research in Quality of Life, 9, 981-995.
Combs, M. A., Canu, W. H., Broman-Fulks, J. J. Rocheleau, C. A., Nieman, D. C. (2012). Perceived stress and ADHD symptoms in adults. Journal of Attention Disorders, 19, 425-434.
Cook, T. D. & Campbell, D. T. (1979). Quasi-experimentation: Design and analysis issues for field settings. Chicago, IL: Rand McNally.
Costello, E., J., Egger, H., & Angold, A. (2005). 10-year research update review: The epidemiology of child and adolescent psychiatric disorders: I. Methods and public health burden. Journal of the American Academy of Child and Adolescent Psychiatry, 44, 972-986.
Costello, E. J., Mustillo, S., Erkanli, A., Keeler, G., & Angold, A. (2003). Prevalence and development of psychiatric disorders in childhood and adolescence. Archives of General Psychiatry, 60, 837-844.
Crichton, A. (1798). An inquiry into the nature and origin of mental derangement: comprehending a concise system of the physiology and pathology of the human mind and a history of the passions and their effects. London: T. Cadell Hr. & W. Davies. (Reprinted by AMS Press, New York, 1976)
Diamond, A. (2005). Attention-deficit disorder (attention-deficit/hyperactivity disorder without hyperactivity): A neurobiologically and behaviorally distinct disorder from attention-deficit/hyperactivity disorder (with hyperactivity). Development and Psychopathology, 17, 807-825.
DuPaul, G, J, & Langberg, J. M. (2015). Educational impairments in children with ADHD. In R. A. Barkley (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th ed.) (pp. 169-190). New York: Guilford Press.
DuPaul,. J., Power, T. J., Anastopoulos, A. D., & Reid, R. (2016). ADHD Rating Scale – 5 for Children and Adolescents: Checklists, Norms, and Clinical Interpretation. New York: Guilford Press.
Fassbender, C., Krafft, C. E., & Schweitzer, J. B. (2015). Differentiating CDS and inattentive symptoms of ADHD using fMRI measures of cognitive control. NeuroImage: Clinical, 8, 390-397.
Flannery, A. J., Luebbe, A. M., & Becker, S. P. (2016). Sluggish cognitive tempo is associated with poorer study skills, more executive functioning deficits, and greater impairment in college students. Journal of Clinical Psychology. Epub ahead of print. doi: 10.1002/jclp.22406
Frazier, T. W., Demaree, H. A., & Youngstrom, E. A. (2004). Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology, 18(3), 543-555.
Garner, A. A., Hansen, A., Baxley, C., Becker, S. P., Sidol, C. A., & Beebe, D. W. (2016). Impact of sleep extension on sluggish cognitive tempo symptoms and driving behavior among adolescents with chronic short sleep. Sleep Medicine, 30, 93-96.
Garner, A. A., Marceaux, J. C., Mrug, S., Patterson, C., & Hodgens, B. (2010). Dimensions and correlates of attention deficit/hyperactivity disorder and sluggish cognitive tempo. Journal of Abnormal Child Psychology, 38, 1097-1107.
Gioia, G. A., Isquith, P. K., Guy, S. C., & Kenworthy, L. (2000). BRIEF: Behavior Rating Inventory of Executive Function – Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc.
Graham, D. M., Crocker, N., Deweese, B. N., Roesch, S. C., Coles, C. D., Kable, J. A., May, P. A., Kalberg, W. O., Sowell, E. R., Jones, K. L., Riey, E. P., Matson, S. N. & the CIFASD (2013). Prenatal alcohol exposure, attention-deficit/hyperactivity disorder, and sluggish cognitive tempo. Alcoholism Clinical and Experimental Research, 37, 338-346.
Harrington, K. M. & Waldman, I. D. (2010). Evaluating the utility of sluggish cognitive tempo in discriminating among DSM-IV subtypes. Journal of Abnormal Child Psychology, 38, 173-184.
Hartman, C. A., Willcutt, E. G., Rhee, S. H., & Penington, B. F. (2004). The relation between sluggish cognitive tempo and DSM-IV ADHD. Journal of Abnormal Child Psychology, 32, 491-503.
Hervey, A. S., Epstein, J. N., & Curry, J. F. (2004). Neuropsychology of adults with attention-deficit/hyperactivity disorder: a meta-analytic review. Neuropsychology, 18(3), 485-503.
Hinshaw, S. P. (2002). Is ADHD an impairing condition in childhood and adolescence? In P. S. Jensen (Ed.), Attention deficit hyperactivity disorder: state of the science – best practices. (pp. 5-1 – 5-21).
Kingston, NJ: Civic Research Institute.
Hoffman, H. (1865). Der Struwwelpeter. Erlangen, Germany: Pestalozzi-Verlag.
Huang-Pollock, C. L., Nigg, J. T., & Carr, T. H. (2005). Deficient attention is hard to find: applying the perceptual load model of selective attention to attention deficit hyperactivity disorder subtypes. Journal of Child Psychology and Psychiatry, 46, 1211-1218.
Jacobson, A. L., Geist, M., & Mahone, M. (2017). Sluggish cognitive tempo, processing speed, and internalizing symptoms: the moderating effect of age. Journal of Abnormal Child Psychology. Epub ahead of print.
Jacobson, A. L., Murphy-Bowman, S. C., Pritchard, A. E., Tart-Zelvin, A., Zabel, T. A., & Mahone, E. M. (2012). Factor structure of a sluggish cognitive tempo scale in clinically-referred children. Journal of Abnormal Child Psychology, 40, 1327-1337.
Jacobson, N. S., Roberts, L. J., Berns, S. B., & McGlinchey, J. B. (1999). Methods for defining ad determining the clinical significance of treatment effects: description, application, and alternatives. Journal of Consult and Clinical Psychology, 67, 300-307.
Jacobson, N. S. & Truax, P. (1991). Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59, 12-19.
Jarrett, M. A., Rapport, H. F., Rondon, A. T., & Becker, S. P. (2014). ADHD dimensions and sluggish cognitive tempo sy,ptoms in relation to self-report and laboratory measures of neuropsychological functioning in college students. Journal of Attention Disorders, 21, 673-683.
Jimenez, A. A., Ballabriga, M. C. J., Martin, A. B., Arrufat, F. J., & Giacobo, R. S. (2013). Executive functioning in children and adolescents with sluggish cognitive tempo and ADHD. Journal of Attention Disorders, 19, 507-514.
Khada, G., Burns, G. L., & Becker, S. P. (2016). Internal and external validity of sluggish cognitive tempo and ADHD Inattention dimensions in teacher ratings of Nepali children. Journal of Psychopathology and Behavioral Assessment, 38, 433-442.
Koriakin, T. A., Mahone, M., & Jacobson, L. A. (2015). Sleep difficulties are associated with parent report of sluggish cognitive tempo. Journal of Developmental and Behavioral Pediatrics, 36, 717-723.
Lahey, B. B., Schaughency, E., Strauss, C., & Frame, C. (1984). Are attention deficit disorders with and without hyperactivity similar or dissimilar disorders? Journal of the American Academy of Child Psychiatry, 23, 302-309.
Langberg, J. M., Becker, S. P., & Dvorsky, M. R. (2014). The association between sluggish cognitive tempo and academic functioning in youth with attention-deficit/hyperactivity disorder (ADHD). Journal of Abnormal Child Psychology, 42, 91-103.
Langberg, J. M., Becker, S. P., Dvorsky, M. R., & Luebbe, A. M. (2014). Are sluggish cognitive tempo and daytime sleepiness distinct constructs? Psychological Assessment, 26, 586-597.
Lee, S. Y., Burns, G. L., & Becker, S. P. (2016). Toward establishing the transcultural validity of sluggish cognitive tempo: Evidence from a sample of South Korean children. Journal of Clinical Child and Adolescent Psychology. Epub ahead of print. http://dx.doi.org/10.1080/15374416.2016.1144192
Lee, S. Y., Burns, G. L., Snell, J., & McBurnett, K. (2013). Validity of the sluggish cognitive tempo symptom dimension in children: Sluggish cognitive tempo and ADHD-inattention as distinct symptom dimensions. Journal of Abnormal Child Psychology, 42, 7-19.
Leikauf, J. E., & Solanto, M. V. (2016). Sluggish cognitive tempo, internalizing symptoms, and executive function in adults with ADHD. Journal of Attention Disorders. Epub ahead of print.
Leopold, D. R., Christopher, M. E, Burns, G. L., Becker, S. P., Olson, R. K., & Willcutt, E. G. (2016). Attention-deficit/hyperactivity disorder and sluggish cognitive tempo throughout childhood: temporal invariance and stability from preschool through ninth grade. Journal of Child Psychology and Psychiatry, 57, 1066-1074.
Lundervold, A. J., Posserud, M. B., Ullebo, A. K., Sorensen, L., & Gillberg, C. (2011). Teacher reports of hypoactivity symptoms reflect slow cognitive processing speed in primary school children. European Child & Adolescent Psychiatry, 20, 121-126.
Markovich-Pilon, A. N., Corkum, P. V., & Joyce, A. M. (2017). Sluggish cognitive tempo: Investigating associated daytime and nighttime impairments. ADHD Report, 25(3), 1-5.
Marshall, S. A., Evans, S. W., Eiraldi, R. B., Becker, S. P., & Power, T. J. (2013). Social and academic impairment in youth with ADHD, predominantly inattentive type and sluggish cognitive tempo. Journal of Abnormal Child Psychology, 42, 77-90.
Mash, E. J., & Barkley, R. A. (2003). Child psychopathology. New York: Guilford.
Maurer, R. G., & Stewart, M. (1980) Attention deficit disorder without hyperactivity in a child psychiatric clinic. Journal of Clinical Psychiatry, 41, 232–233.
McBurnett, K., Clemow, D., Williams, D., Villodas, M., Wietecha, L., & Barkley, R. (2017). Atomoxetine-related change in sluggish cognitive tempo is partially independent of change in attention-deficit/hyperactivity disorder inattentive symptoms. Journal of Child and Adolescent Psychopharmacology, 27, 38-42.
McBurnett, K., Pfiffner, L. J., & Frick, P. J. (2001). Symptom properties as a function of ADHD type: an argument for continued study of sluggish cognitive tempo. Journal of Abnormal Child Psychology, 29, 207-213.
McBurnett, K., Villodas, M., Burns, G. L., Hinshaw, S. P., Beaulieu, A., & Pfiffner, L. J. (2014). Structure and validity of sluggish cognitive tempo using an expanded item pool in children with attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology, 42, 37-48.
McConaughy, S. H., & Achenbach, T. M. (2001). Child Behavior Checklist – Direct Observation Form. Burlington, VT: Authors, University of Vermont.
McConaughy, S. H., Ivanova, M., Antshel, K., & Eiraldi, R. B. (2009). Standardized observational assessment of Attention Deficit/Hyperactivity Disorder Combined and Predominantly Inattentive Subtypes: I. Test session observations. School Psychology Review, 38, 45-66.
McConaughy, S. H., Ivanova, M., Antshel, K., Eiraldi, R. B., & Dumenci, L. (2009). Standardized observational assessment of Attention Deficit/Hyperactivity Disorder Combined and Predominantly Inattentive Subtypes: II. Classroom observations. School Psychology Review, 39, 362-381.
McGrath, L. M., Hutaff-Lee, C., Scott, A., Boada, R., Shriberg, L. D. & Pennington, B. F. (2008). Children with comorbid speech sound disorder and specific language impairment are at increased risk for attention-deficit/hyperactivity disorder. Journal of Abnormal Child Psychology, 36, 151-163.
McQuade, J. D. & Hoza, B. (2015). Peer relationships of children with ADHD. In R. A. Barkley (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th Ed.)(pp. 210-222). New York: Guilford Press.
Mikami, A. Y., Huang-Pollack, C. L., Pfiffner, L. J., McBurnett, K., & Hangai, D. (2007). Social skills differences among attention-deficit/hyperactivity disorder types in a chat room assessment task. Journal of Abnormal Child Psychology, 35, 509-521.
Milich, R., Ballentine, A.C., & Lynam, D.R. (2001). ADHD/combined type and ADHD/predominantly inattentive type are distinct and unrelated disorders. Clinical Psychology: Science and Practice, 8, 463-488.
Mirsky, A. F. (1996). Disorders of attention. In R. G. Lyon & N. A. Krasnegor (Eds.), Attention, memory, and executive function (pp. 71-96). Baltimore, MD: Paul H. Brookes.
Mitchell, J. T., Knouse, L. E., Nelson-Gray, R. O., & Kwapil, T. R. (2009). Self-reported ADHD symptoms among college students: item position affects symptom endorsement rates. Journal of Attention Disorders, 13, 154-160.
Moruzzi, S., Rijsdijk, F., & Battaglia, M. (2014). A twin study of the relationships among inattention, hyperactivity-impulsivity and sluggish cognitive tempo problems. Journal of Abnormal Child Psychology, 42, 63-75.
Mueller, A. K., Tucha, L., Koerts, J., Groen, Y., Lange, K. W., Tucha, O. (2014). Sluggish cognitive tempo and its neurocognitive, social, and emotional correlates: a systematic review of the current litearature. Journal of Molecular Psychiatry, 2, 5.
Olfson, M., Marcus, S. C., Weissman, M. M., & Jensen, P. S. (2002). National trends in the use of psychotropic medications by children. Journal of the American Academy of Child and Adolescent Psychiatry, 41, 514-521.
Palmer, E. D. & Finger, S. (2001). An early description of ADHD (Inattentive Subtype): Dr. Alexander Crichton and "Mental Restlessness" (1798). Child Psychology and Psychiatry Review, 6, 66-73.
Penny, A. M., Waschbusch, D. A., Klein, R. M., Corkum, P., & Eskes, G. (2009). Developing a measure of sluggish cognitive tempo for children: Content validity, factor structure, and reliability. Psychological Assessment, 21, 380-389.
Pfiffner, L. J., Mikami, A. Y., Huang-Pollock, C., Easterlin, B., Zalecki, C., & McBurnett, K. (2007). A randomized, controlled trial of integrated home-school behavioral treatment for ADHD, Predominantly Inattentive Type. Journal of the American Academy of Child and Adolescent Psychiatry, 48, 1041-1050.
Pliszka, S. (2015). Comorbid psychiatric disorders in children with ADHD. In R. A. Barkley (Ed.) Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th ed.) (pp. 140-168). New York: Guilford Press.
Preszler, J., Burns, G. L., Litson, K., Geiser, C., Servera, M., & Becker, S. P. (2017). How consistent is sluggish cognitive tempo across occasions, sources, and settings? Evidence from latent state-trait modeling. Assessment. Epub ahead of print.
Raiker, J. S., Greening, L., Stoppelbein, L., Becker, S. P., Fite, P. J., & Luebbe, A. M. (2015). Mediating effect of psychopathy on the risk of social problems among children with ADHD versus sluggish cognitive tempo symptoms. Child Psychiatry and Human Development, 46, 523-532.
Reeves, C. B., Palmer, S., Gross, A. M., Simonian, S. J., Taylor, L., Willigham, E., & Mulhern, R. K. (2007). Brief report: Sluggish cognitive tempo among pediatric survivors of acute lymphoblastic leukemia. Journal of Pediatric Psychology, 32, 1050-1054.
Reynolds, C., & Kamphaus, R. (2004). Behavioral Assessment System for Children - II. Circle Pines, MN: American Guidance Service.
Roberts, W., Milich, R., & Barkley, R. A. (2015). Primary symptoms, diagnostic criteria, subtyping, and prevalence of ADHD. In R. A. Barkley (Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatment (4th edition)(pp. 51-80). New York: Guilford Press.
Rowe, R., Maughan, B., & Goodman, R. (2004). Childhood psychiatric disorder and unintentional injury: Findings from a national cohort study. Journal of Pediatric Psychology, 29, 119-130.
Saxbe, C. & Barkley, R. A. (2014). The other attention disorder? Sluggish cognitive tempo vs. ADHD: Update for clinicians. Journal of Clinical Psychiatry, 20, 38-49.
Servera, M., Bernad, M., Carillo, J. M., Collado, S., & Burns, G. L. (2016). Longitudinal correlates of sluggish cognitive tempo and ADHD-inattention symptom dimensions with Spanish children. Journal of Clinical Child and Adolescent Psychology, 45, 632-641.
Shelton, C. R., Addison, W. E., & Hartung, C. M. (2017). ADHD and SCT symptomatology in relation to college students’ use of self-regulated learning strategies. Journal of Attention Disorders. Epub ahead of print.
Skirbekk, B., Hansen, B. H., Oerbeck, B., & Kristensen, H. (2011). The relationship between sluggish cognitive tempo, subjects of attention-deficit/hyperactivity disorder, and anxiety disorders. Journal of Abnormal Child Psychology, 39, 513-525.
Smallwood, J., Fishman, D., & Schooler, J. (2007). Counting the cost of an absent mind: Mind wandering as an under recognized influence on educational performance. Psychonomic Bulletin & Review, 14, 230-236.
Smallwood, J. & Schooler, J. (2006). The restless mind. Psychological Bulletin, 132, 946-958.
Smith, Z. R. & Langberg, J. M. (2017). Predicting academic impairment and internalizing psychopathology using a multi-dimensional framework of Slluggish Cognitive Tempo with parent- and adolescent reports. European Child and Adolescent Psychiatry. Epub ahead of print.
Tamm, L., Brenner, S. B., Bamberger, M. E., & Becker, S. P. (2016). Are sluggish cognitive tempo symptoms associated with executive functioning in preschoolers? Child Neuropsychology, Epub ahead of print.
Tamm, L., Garner, A. A., Loren, R. E. A., Epstein, J. N., Vaughn, A. J., Ciesielski, H. A., & Becker, S. P. (2016). Slow sluggish cognitive tempo symptoms are associated with poorer academic performance in children with ADHD. Psychiatry Research, 242, 251-259.
Thomas, C. P., Conrad, P., Casler, R., & Goodman, E. (2006). Trends in the use of psychotropic medications among adolescents, 1994-2001. Psychiatric Services, 57, 63-69.
Thorell, L. B., & Nyberg, L. (2008). The Childhood Executive Functioning Inventory (CHEXI): A new rating instrument for parents and teachers. Developmental Neuropsychology, 33, 536-552.
Todd, R. D., Rasmussen, E. R., Wood, C., Levy, F., & Hay, D. (2004). Should sluggish cognitive tempo symptoms be inclludedin the diagnosis of attention-deficit/hyperactivity disorder? Journal of the American Academy of Child and Adolescent Psychiatry, 43, 588-597.
Toplak, M. E., West, R., F., & Stanovich, K. E. (2013). Practitioner Review: Do performance-based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry, 54, 113-224.
Wahlstedt, C. & Bohlin, G. (2010). DSM-IV defined inattention and sluggish cognitive tempo: independent and interactive relations to neuropsychological factors and comorbidity. Child Neuropsychology, 16(4), 250-365.
Watabe, Y., Owens, J. S., Evans, S. W., & Brandt, N. E. (2013). The relationship between sluggish cognitive tempo and impairment in children with and without ADHD. Journal of Abnormal Child Psychology, 42, 105-115.
Weinberg, W. A., & Brumback, R. A. (1990). Primary disorder of vigilance: a novel explanation of inattententiveness, daydreaming, boredom, restlessness, and sleepiness. Journal of Pediatrics, 116, 720–725.
Weinberg, W. A., & Brumback, R. A. (1992). The myth of attention deficit-hyperactivity disorder: Symptoms resulting from multiple causes. Journal of Child Neurology, 7, 431-445.
Weinberg, W. A. & Harper, C. R. (1993). Vigilance and its disorders. Neurology Clinics, 11, 59-78.
Wietecha, L., Williams, D., Shaywitz, S., Shaywitz, B., Hooper, S. R., Wigal, S. B., Dunn, D., & McBurnett, K. (2013). Atomoxetine improved attention in children and adolescents with attention-deficit/hyperactivity disorder and dyslexia in a 16 week, acute, randomized, double-blind trial. Journal of Child and Adolescent Psychopharmacology, 23, 605-609.
Willcutt, E. G. (2015). Theories of ADHD. In R. A. Barkley Ed.), Attention deficit hyperactivity disorder: A handbook for diagnosis and treatmet (3rd ed.)(391-404). New York: Guilford Press.
Willcutt, E. G., Chhabildas, N., Kinnear, M., DeFries, J. C., Olson, R. K., Leopold, D. R., Keenan, J. M., & Pennington, B. F. (2013). The internal and external validity of sluggish cognitive tempo and its relation with DSM-IV ADHD. Journal of Abnormal Child Psychology, 42, 21-35.
Willcutt, E. G., Doyle, A. E., Nigg, J. T., Faraone, S. V., & Pennington, B. F. (2005). Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry, 57, 1336-1346.
Willcutt, E. G., Nigg, E. T., Pennington, B. F., Solanto, M. V., Rohde, L. A., Tannock, R., Loo, S. K., Carlson, C. L., McBurnett, K., & Lahey, B. B. (2012). Validity of the DSM-IV attention deficit/hyperactivity disorder symptom dimensions and subtypes. Journal of Abnormal Psychology, 121, 991-1010.
Wood, W. L. M., Lewandowski, L. J., Lovett, B. J., & Antshel, K. M. (2017). Executive dysfunction and functional impairment associated with sluggish cognitive tempo in emerging adulthood. Journal of Attention Disorders, 21, 691-700.
Wood, W. L. M., Potts, H. E., Lewandowski, L. J., & Lovett, B. J. (2016). Sluggish cognitive tempo and speed of performance. Journal of Attention Disorders, 21, 684-690.


 ContinuingEdCourses.Net is approved by the American Psychological Association (APA) to sponsor continuing education for psychologists. ContinuingEdCourses.Net maintains responsibility for this program and its content.
ContinuingEdCourses.Net is approved by the American Psychological Association (APA) to sponsor continuing education for psychologists. ContinuingEdCourses.Net maintains responsibility for this program and its content. ContinuingEdCourses.Net, provider #1107, is approved as an ACE provider to offer social work continuing education by the Association of Social Work Boards (ASWB) Approved Continuing Education (ACE) program. Regulatory boards are the final authority on courses accepted for continuing education credit. ACE provider approval period: 3/9/2005-3/9/2027.
ContinuingEdCourses.Net, provider #1107, is approved as an ACE provider to offer social work continuing education by the Association of Social Work Boards (ASWB) Approved Continuing Education (ACE) program. Regulatory boards are the final authority on courses accepted for continuing education credit. ACE provider approval period: 3/9/2005-3/9/2027. ContinuingEdCourses.Net has been approved by NBCC as an Approved Continuing Education Provider, ACEP No. 6323. Programs that do not qualify for NBCC credit are clearly identified. ContinuingEdCourses.Net is solely responsible for all aspects of the programs.
ContinuingEdCourses.Net has been approved by NBCC as an Approved Continuing Education Provider, ACEP No. 6323. Programs that do not qualify for NBCC credit are clearly identified. ContinuingEdCourses.Net is solely responsible for all aspects of the programs. ContinuingEdCourses.Net is recognized by the New York State Education Department's State Board for Psychology (NYSED-PSY) as an approved provider of continuing education for licensed psychologists #PSY-0048.
ContinuingEdCourses.Net is recognized by the New York State Education Department's State Board for Psychology (NYSED-PSY) as an approved provider of continuing education for licensed psychologists #PSY-0048.






